Degradation reactions, their reaction enthalpy (blue = endothermic, red = exothermic) and the temperature range at which they are observed in differential scanning calorimetry, accelerated rate calorimetry or electrochemical measurements. Products that are also reactants are highlighted in bolda.
| Name | Abbr. | Equation | ΔrH̲/kJ mol−1 | T Start/°C |
|---|---|---|---|---|
| Conductive salt decomposition29 | CSD | LiPF6 ⇌ LiF + PF5 |
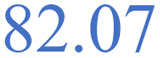
|
25*/60–80 |
| PF5 decomposition29 | PFD | PF5 + H2O ⇌ 2HF + POF3 |
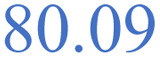
|
25*/60–80 |
| POF3 decomposition29 | POFD | POF3 + H2O → HF + HPO2F2 |
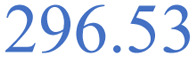
|
25*/60–80 |
| Organic SEI production30,31 | OSP | 2LiC6 + 2C3H4O3 (EC) → (CH2OCO2Li)2 + C2H4 + 2C6 |

|
25**/80–120 |
| Inorganic SEI production30,31 | ISP | 2LiC6 + C3H4O3 (EC) → Li2CO3 + C2H4 + 2C6 |
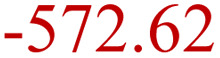
|
25**/80–120 |
| LiOH production20 | LSP | LiC6 + H2O → LiOH + 0.5H2 + C6 |

|
25**/80–120 |
| Organic SEI decomposition5,31 | OSD | (CH2OCO2Li)2 → Li2CO3 + C2H4 + CO2 + 0.5O2 |

|
60–120 |
| Inorganic SEI decomposition32 | ISD | Li2CO3 + 2HF → 2LiF + H2O + CO2 |
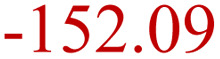
|
25 |
| LiOH decomposition33 | LSD | LiC6 + LiOH → Li2O + 0.5H2 + C6 |
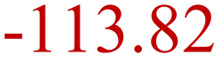
|
100–120 |
| Cathode decomposition34 | CD |
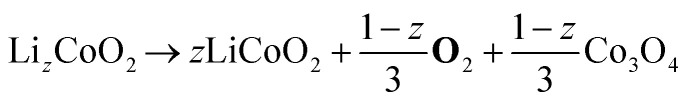
|
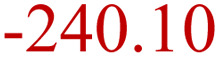
|
150–220 |
| EMC combustion34 | EMCD | 3.5O2 + C4H8O3 (EMC) → 4CO2 + 4H2O |

|
180–350 |
| EC combustion34 | ECD | 2.5O2 + C3H4O3 (EC) → 3CO2 + 2H2O |

|
180–350 |
* slow process during cell formation; ** process considered indirectly.
