Abstract
Objectives
This study examined the malleability of a tripartite cluster of purported mechanistic variables targeted in a 4-week intervention program designed to improve adults’ engagement in physical activity. The targeted cluster of purported mechanisms consisted of negative views of aging (NVOA), self-efficacy beliefs, and behavioral intentions.
Methods
A randomized single-blind control group design was used to implement the AgingPLUS program in a sample of middle-aged and older adults (N = 335; Mage = 60.1 years; SDage = 8.3 years; age range 45–75 years).
Results
Using an intention-to-treat approach and analyses of covariance adjusting for participants’ baseline scores, findings showed significant improvements in the purported mechanistic variables both at the immediate (Week 4) and delayed posttest (Week 8) in the treatment group but not in the control group. These improvements were for the most part maintained until 8-month postrandomization, although to a smaller extent. Specifically, participants in the AgingPLUS group showed significant improvements in NVOA, self-efficacy beliefs, and behavioral intentions compared to the Health Education control group. Standardized effect sizes for statistically significant effects were variable and ranged from small (d = −0.23) to large (d = 0.80). Effect sizes showed some decay of the intervention at the 8-month posttest.
Discussion
Taken together, the findings supported the efficacy of the AgingPLUS program and showed that variables representing the purported mechanisms of the intervention were significantly moved in a positive direction. In doing so, the AgingPLUS program met a major requirement of the experimental medicine approach to behavior change interventions.
Clinical Trials Registration Number
NCT0329948
Keywords: Negative views of aging, self-efficacy beliefs, behavioral intentions, experimental medicine approach
Although it is well documented that certain health behaviors (e.g., eating a healthy diet; engaging in physical activity) can promote healthy aging, the mechanisms through which these behaviors can be promoted are less well understood (Lachman et al., 2018; Nielsen & Reiss, 2012). This has led intervention researchers to conclude that it is essential to address the social-cognitive and motivational barriers that hinder adults from engaging in more positive lifestyle behaviors (Ma et al., 2016; Nielsen & Reiss, 2012). Following the experimental medicine approach (Riddle, 2015), the present study examined the malleability of a tripartite cluster of such social-cognitive and motivational factors, namely negative views of aging (NVOA), self-efficacy beliefs, and behavioral intentions. Thus, this study focused on the first necessary step of the experimental medicine approach and examined whether a 4-week intervention program designed to increase sedentary adults’ engagement in physical activity changed the purported mechanisms of the intervention (see also Sheeran et al., 2016).
Negative Views of Aging
Although adults’ views of their own aging may include both positive and negative perceptions, in the general population, most general and personal views of aging (VOA) tend to be negative (Lindland et al., 2016). For the purposes of this study, we define NVOA as individuals’ negative age stereotypes, negative attitudes, and negative self-perceptions about growing old(er) and about old people as a social group (Diehl et al., 2021). NVOA predict a host of negative physical and cognitive outcomes (for an overview, see Diehl et al., 2021). These effects of NVOA have been shown in experimental (e.g., Levy, 1996) and quasi-experimental studies (e.g., Levy et al., 2002). For example, in one of the first experimental studies, Levy (1996) showed that priming older adults with negative age words resulted in a significant worsening in memory performance compared to priming with positive age words. Similarly, findings from quasi-experimental studies have shown that NVOA, including negative self-perceptions of aging (SPA), are associated with less engagement in preventive health care (Levy & Myers, 2004), poorer cognitive functioning and steeper cognitive decline (Siebert et al., 2018a), poorer physical health and functional status (Sargent-Cox et al., 2012), and reduced longevity (Levy et al., 2002).
Conversely, another body of research has shown that promoting positive views of aging (PVOA) tends to be associated with positive outcomes, including engagement in preventive health behavior (Levy & Myers, 2004), better physical and mental health (Wolff et al., 2014), and increased longevity (Wurm & Schäfer, 2022). Thus, these findings support the assumption that adults’ VOA may play a causal role in shaping adults’ course of aging and major developmental outcomes. Moreover, this evidence suggests that (a) NVOA as habitual patterns of negative thought undermine adults’ motivation and behavior and (b) promoting PVOA may be an avenue to motivate behavior that could support positive aging.
A major problem, however, is that to date, only a limited number of studies have examined the malleability of adults’ NVOA and have reported mixed results (Beyer et al., 2019; Nehrkorn-Bailey et al., 2023; Sarkisian et al., 2007; Wolff et al., 2014). Thus, this study examined whether adults’ NVOA could be altered to become more positive with the intention to motivate greater engagement in physical activity.
Self-Efficacy Beliefs
A second motivational barrier often discussed in the context of behavior change is individuals’ low perceived control and low self-efficacy beliefs (Bandura, 1997; Lachman et al., 2018; Schwarzer, 2008; see Author Note 1). Both concepts refer to individuals’ beliefs about the likelihood that their actions can bring about desired outcomes. Although the literature on the development of control and self-efficacy beliefs across adulthood indicates that individuals’ sense of personal control and self-efficacy tends to decline as they grow older (Krause, 2007; Robinson & Lachman, 2017), it is also well documented that a greater sense of control and self-efficacy is associated with numerous positive outcomes. For example, several studies have shown that individuals who had a greater sense of personal control maintained their cognitive and functional health longer and more effectively (Lachman & Agrigoroaei, 2010; Parisi et al., 2017), were more likely to lead a healthy lifestyle, and were less likely to become disabled (Robinson & Lachman, 2017). Conversely, adults with a lower sense of control and self-efficacy may be at an increased risk for a variety of negative behavioral, affective, and functional outcomes, including higher levels of depression, anxiety, stress, and poorer memory performance (Lachman et al., 2011). Moreover, it has been documented that individuals with lower control and self-efficacy beliefs are less likely to (a) enroll in health change programs and (b) maintain newly acquired health behaviors over time (Bandura, 1997; Schwarzer, 2008).
Although one meta-analysis (Sheeran et al., 2016) showed significant changes in self-efficacy for a variety of interventions, there is still an ongoing debate about the magnitude of the malleability of self-efficacy beliefs in middle-aged and older adults and how improvements in self-efficacy may be maintained over time (Ashford et al., 2010). West et al. (2008) showed that participants in the training group of a memory training program improved their memory self-efficacy compared to the participants in the wait-list control group after completion of the program. Similarly, Lachman and colleagues (2006) found that encouraging strategy use during a memory training program resulted in higher self-efficacy in older adults, but not in young or middle-aged adults. French et al.’s (2014) literature review identified behavior change techniques that may increase adults’ self-efficacy beliefs regarding the adoption of physical activity behavior. These authors found that teaching self-regulatory strategies was associated with improved self-efficacy beliefs after the interventions. The meta-analysis by Sheeran et al. (2016) mostly confirmed these findings but also pointed out that only limited knowledge exists about the long-term maintenance of the observed improvements. In summary, there is evidence suggesting that adults’ personal control and self-efficacy beliefs can be strengthened through focused interventions (Ashford et al., 2010; Prestwich et al., 2014). However, there also remain several unresolved issues, including the long-term maintenance of the obtained improvements and the strength of association with behavioral outcomes (Sheeran et al., 2016).
Behavioral Intentions
Even if adults develop more positive VOA and a stronger sense of self-efficacy, another obstacle to the initiation and maintenance of behavior change often lies in individuals’ weak behavioral intentions (i.e., self-instructions to perform a particular behavior) and lack of action-planning skills (Nielsen & Reiss, 2012; Schwarzer, 2008; Sheeran & Webb, 2016). Adults with weak behavioral intentions have been shown to engage in fewer health-promoting behaviors (Sheeran & Webb, 2016) and often give up quickly when facing obstacles or being challenged to maintain a certain behavior over a longer time (Nielsen & Reiss, 2012; Schwarzer, 2008). Thus, researchers have suggested that strengthening individuals’ behavioral intentions and teaching more effective action planning may be another critical component for initiating and maintaining behavior change (Schwarzer, 2008; Sheeran & Webb, 2016).
Although it is well documented that behavioral intentions alone are not sufficient for goal achievement and long-term behavior change (Sheeran & Webb, 2016), there is also evidence showing that behavioral intentions are critical for the development and maintenance of action plans and the subsequent adoption of new behavior (Schwarzer, 2008). Therefore, individuals’ behavioral intentions are considered a critically important component of the motivational stage of behavior change (Schwarzer, 2008). Focusing on individuals’ behavioral intentions in combination with their self-efficacy beliefs and action-planning skills is also supported by findings from a meta-analysis showing that medium-to-large changes in intention led to small-to-medium changes in behavior (Webb & Sheeran, 2006).
The Present Study
The present study examined the malleability of middle-aged (age range 45–59 years) and older adults’ (age range 60–75 years) NVOA, self-efficacy beliefs, and behavioral intentions as part of the AgingPLUS randomized controlled trial (RCT; Diehl et al., 2020). Although engagement in physical activity was the behavioral outcome of the RCT, following the experimental medicine approach (Riddle, 2015), this study focused on the first necessary proof in the RCT. That is, this study tested whether the treatment condition of the trial was successful in changing the social-cognitive and motivational mechanisms that were hypothesized causing the change in the target outcome (Sheeran et al., 2016). Thus, we tested the following hypothesis: At the immediate (Week 4), the delayed (Week 8), and the long-term posttest (8-month postrandomization), participants in the AgingPLUS group will show significant (p < .05) improvements in NVOA, self-efficacy beliefs, and behavioral intentions compared to baseline and compared to the participants in the active control group.
Method
The Institutional Review Board (IRB) of Colorado State University approved all components of the trial. All study procedures adhered to the Consolidated Standards of Reporting Trials (CONSORT; Moher et al., 2010) guidelines to assure the scientific rigor of the trial and the reproducibility of the protocol and findings.
Participants and Recruitment
Study participants in the age range from 45 to 75 years were recruited via e-mail announcements and study flyers; the article by Diehl et al. (2020) provides a detailed justification of the chosen age range. Middle-aged participants were primarily recruited through an employee listserv at Colorado State University. Older adults were recruited from preexisting participant registries, senior centers, and local faith-based and civic organizations. Trained research assistants screened interested individuals for eligibility. The eligibility criteria (i.e., inclusion and exclusion criteria) and screening procedures are described in detail in Diehl et al. (2020). All participants came from a tri-county area in the northern Colorado Front Range.
After initial screening for eligibility, 402 out of 549 adults aged 45–75 were invited to participate in the study. Upon completion of the baseline assessment, a total of 335 participants were randomly assigned to either the treatment group (n = 173) or the active control group (n = 162). Estimation of the required sample size was based on an a priori power analysis for mixed linear models (Maas & Hox, 2005). This power analysis resulted in a total sample size of 300 participants for a study with (a) two groups at Level 2 (i.e., treatment conditions) and four times of measurement at Level 1 (Baseline, Week 4, Week 8, 8-month posttest); (b) an anticipated attrition rate of 20%; and (c) a power of 0.80 to detect medium effect sizes at a significance level of α = 0.05 (see Diehl et al., 2020). Figure 1 shows the CONSORT diagram for the trial. Table 1 shows the sociodemographic characteristics of the study sample.
Figure 1.
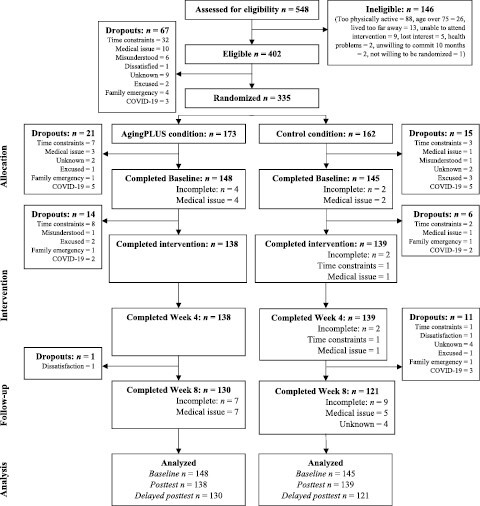
CONSORT diagram describing the participant flow through the study. CONSORT = Consolidated Standards of Reporting Trials; COVID-19 = coronavirus disease 2019.
Table 1.
Sample Characteristics by Intervention Group
| Variable | AgingPLUS group (n = 173) | Health Education group (n = 162) | p Value |
|---|---|---|---|
| Age, mean (SD) | 60.0 (8.4) | 60.1 (8.2) | .92 |
| Age, n (%) | .99 | ||
| 45–54 | 53 (30.6) | 48 (29.6) | |
| 55–64 | 66 (38.2) | 64 (39.5) | |
| 65–75 | 54 (31.2) | 50 (30.9) | |
| Women, n (%) | 142 (82.1) | 138 (85.2) | .44 |
| White, n (%) | 141 (81.5) | 143 (88.3) | .36 |
| Marital status, n (%) | .29 | ||
| Single | 21 (12.1) | 14 (8.6) | |
| Married/committed partnership | 105 (60.7) | 92 (56.8) | |
| Divorced/separated | 31 (17.9) | 40 (24.7) | |
| Widowed | 11 (6.4) | 14 (8.6) | |
| Education, n (%) | .76 | ||
| High school | 23 (13.3) | 28 (17.3) | |
| Associate’s degree | 13 (7.5) | 15 (9.3) | |
| Bachelor’s degree | 59 (34.1) | 46 (28.4) | |
| Master’s degree | 59 (34.1) | 56 (34.6) | |
| Doctoral degree | 15 (8.7) | 15 (9.3) | |
| Education in years, mean (SD) | 17.5 (2.8) | 17.4 (2.6) | .73 |
| Income, n (%) | .57 | ||
| <$35,000 | 33 (19.1) | 26 (16.1) | |
| $35,000–79,999 | 40 (23.1) | 33 (20.4) | |
| $80,000–99,999 | 37 (21.4) | 37 (22.8) | |
| ≥$100,000 | 50 (28.9) | 58 (35.8) | |
| Employment, n (%) | .64 | ||
| Employed full-time | 72 (41.6) | 71 (43.8) | |
| Employed part-time | 28 (16.2) | 25 (15.4) | |
| Retired | 53 (30.6) | 48 (29.6) | |
| Other | 15 (8.7) | 16 (9.9) |
Notes: SD = standard deviation. The employment category “Other” includes participants who were in bridge/temporary jobs, pursued a second career, or were unemployed.
Although major efforts were made to oversample men to achieve an equal distribution of men and women in the study, most participants were women (see Table 1). Most of the participants were married and White. The sample had a high level of education, with an average of 17.45 years of schooling (standard deviation [SD] = 2.74 years) and reported being overall in good health (M = 4.51, SD = 1.04; 1 = Very poor, 6 = Excellent). To ensure that the treatment group (i.e., AgingPLUS program) and the active control group (i.e., Health Education program) were balanced by gender and age group, the randomization to group was stratified by gender and age (45–54, 55–64, and 65–75 years). As Table 1 shows, the AgingPLUS and Health Education groups did not differ in terms of age composition and the distribution of gender, race/ethnicity, marital status, education levels, income, or employment status (all ps > .05).
Procedure
The study used a randomized, single-blind pretest–posttest active control group design to examine the efficacy of the AgingPLUS program against a generic health education program known as 10 Keys to Healthy Aging (Newman et al., 2010). The intervention was administered across four educational sessions during Weeks 1–4. NVOA, self-efficacy beliefs, and behavioral intentions were assessed at baseline (Week 0), immediate posttest (Week 4), delayed posttest (Week 8), and a long-term posttest 8-month postrandomization (i.e., 6 months after Week 8).
Baseline assessment (Week 0)
Eligible adults completed a baseline assessment comprised of a psychosocial assessment session, a week of physical activity monitoring, and a physical assessment session. During the psychosocial assessment session, participants completed a comprehensive psychosocial questionnaire, including the measures of NVOA, self-efficacy beliefs, and behavioral intentions. Participants were also instructed on how to use a daily activity log (DAL) and how to wear an ActiGraph (ActiGraph, LLC, Pensacola, FL) accelerometer to monitor their physical activity for 7 days following the session. During the monitoring period, research staff made two check-in calls to answer any questions and sent daily text messages reminding participants to complete the DAL. After completion of the baseline session, participants were randomized to either the AgingPLUS group (i.e., treatment group) or the Health Education group (i.e., active control group).
At the end of the week following the psychosocial assessment, participants returned the DAL and accelerometer at their physical assessment session. The physical assessment session consisted of height, weight, and waist circumference measurements, blood pressure readings, a submaximal cardiorespiratory fitness test performed on a stationary bike, and the Short Physical Performance Battery (Guralnik et al., 1994).
Intervention sessions (Weeks 1–4)
During Weeks 1–4, participants attended weekly, 2-hr long sessions in small groups of 5–15 participants. A detailed description of the session content for the AgingPLUS and the Health Education program (Newman et al., 2010) can be found in Supplementary Appendix. Both programs were presented by trained and certified group facilitators, required in-person attendance, and occurred on independent days to avoid contact between groups. Implementation fidelity of the programs was monitored by videotaping each class session and having the videotapes reviewed by an independent intervention expert (G. W. Rebok).
Immediate (Week 4), delayed (Week 8), and long-term posttest (Month 8)
At the end of the fourth intervention session when all content in the treatment and control group had been presented, participants were reassessed with the same psychosocial questionnaire that had been administered at baseline (i.e., immediate posttest). One month after the completion of the intervention (Week 8), all participants completed another follow-up assessment with the same measures and format as at baseline (i.e., delayed posttest). In addition, at Week 8, a new activity monitoring week and physical assessment session were conducted. Participants in the AgingPLUS group were required to specify their self-chosen physical activity goals in the DAL prior to starting the 7-day monitoring period. The DAL and actigraph were returned by participants at the Week 8 physical assessment. The Week 8 assessment procedures were repeated at the long-term posttest 8-month postrandomization.
Pandemic-related adjustments in procedures
Halfway through the RCT, the university and IRB mandated adjustments to the research protocol due to the start of the coronavirus disease 2019 (COVID-19) pandemic (i.e., the State of Colorado went in mandated lockdown on March 25, 2020). The following adjustments were implemented to ensure the safety of the study participants and research staff: (1) performing COVID-19 screenings; (2) halting intervention groups during the lockdown period (March–June 2020); (3) administering questionnaires as electronic forms during the lockdown period; (4) postponing the Week 8 assessments until after the lockdown period; (5) administration of questionnaires as take-home packets during the high-risk period (i.e., before COVID vaccines were widely available; July 2020 to June 2021); (6) limiting educational groups to 5–8 participants; and (7) mandating the use of N95-quality face masks at all in-person meetings. The onset of the COVID-19 pandemic also resulted overall in slower enrollment and greater attrition for the remainder of the trial.
Measures
Views of aging
General views of aging
Three measures were used to assess general VOA: The Age Stereotypes Scale (AS; Kornadt & Rothermund, 2011), the Expectations Regarding Aging (ERA) questionnaire (Sarkisian et al., 2005), and the short version of the Essentialist Beliefs About Aging scale (EBA-S; Weiss & Diehl, 2021).
The AS consists of 27 items measuring a person’s general age stereotypes across eight domains: family and partnership, physical and mental fitness, health and appearance, friends and acquaintances, work and employment, religion and spirituality, personality and way of living, leisure activities and social or civic commitment, and financial situation. Each domain is assessed with 3–5 items, with participants placing their answers on an 8-point scale between a negative and positive pole. A total age stereotypes score was calculated by summing the mean domain scores and dividing the sum by the number of domains (i.e., mean total score). A higher score indicates more positive age stereotypes. The reliability coefficients (Cronbach’s α) ranged from 0.83 to 0.91 across occasions of measurement and across intervention groups.
The short ERA questionnaire consists of 12 items assessing participants’ expectations regarding positive or negative age-related changes. Items are answered on a 4-point scale (1 = Definitely true; 4 = Definitely false) and a total score is calculated, with higher scores indicating more positive expectations regarding aging. The reliability coefficients ranged from α = 0.84 to 0.88.
The short EBA-S was used to assess participants’ general beliefs regarding the fixed or malleable nature of aging. The short version has four items which participants answer on a 6-point scale (1 = Do not agree; 6 = Absolutely agree). The reliability coefficients were acceptable across assessments and intervention groups, ranging from α = 0.62 to 0.74.
Personal Views of Aging
Participants’ personal VOA were measured by the 10-item Awareness of Age-Related Change (AARC) questionnaire (Kaspar et al., 2019). Using a 5-point scale (1 = Not at all; 5 = Very much), the items ask participants about positive (i.e., AARC-gains) and negative (i.e., AARC-losses) SPA. The reliability coefficients for the AARC-losses subscale ranged from α = 0.74 to 0.79 across occasions of measurement and intervention groups; the corresponding coefficients for the AARC-gains subscale ranged from α = 0.57 to 0.69.
Self-efficacy beliefs
General self-efficacy beliefs
The General Self-Efficacy scale (GSE; Schwarzer & Jerusalem, 1995) and the Self-Regulation Scale (SRS; Diehl et al., 2006) were used to assess participants’ general self-efficacy beliefs. The GSE (Schwarzer & Jerusalem, 1995) consists of eight items that measure participants’ general sense of self-efficacy. Items are answered on a 4-point scale (1 = Not at all true; 4 = Completely true) and higher scores indicate greater general self-efficacy beliefs. The reliability coefficients ranged from α = 0.84 to 0.89.
The 10-item SRS (Diehl et al., 2006) assesses participants’ self-regulatory abilities in different situations. Items are answered on a 4-point scale (1 = Not at all true; 4 = Completely true) and higher scores indicate greater general self-regulation abilities. The reliability coefficients ranged from α = 0.82 to 0.89 across occasions of measurement and intervention groups.
Domain-specific self-efficacy beliefs
Three domain-specific measures of self-efficacy were included: motivational self-efficacy (MSE), volitional self-efficacy (VSE), and walking self-efficacy (WSE). The three-item MSE measure (Schwarzer, 2008) asks participants about their confidence to start a physical activity routine in the future. VSE was also assessed with three items (Schwarzer, 2008), measuring participants’ confidence to maintain a future physical activity routine. For both measures, participants responded on a 6-point scale (1 = Totally disagree; 6 = Totally agree) with higher scores indicating greater motivational or volitional self-efficacy, respectively. Reliabilities ranged from α = 0.87 to 0.94 for MSE and from α = 0.88 to 0.94 for VSE.
The WSE scale (McAuley et al., 2000) was used to assess participants’ confidence to successfully engage in 5-min periods of walking at a moderately fast pace, starting at 5 min and ending with 50 min. For each 5-min increase in walking time, participants report their confidence on a 100-percent scale in 10-percent increments (i.e., ranging from 0% to 100%). The average confidence rating is calculated across the 10 items and a higher number indicates higher walking self-efficacy. Reliability coefficients ranged from 0.97 to 0.98.
Behavioral intentions
Behavioral intentions were assessed in terms of participants’ exercise intentions (Schwarzer, 2008). The measure has three items to which participants responded on a 6-point scale (1 = Not at all true; 6 = Absolutely true). The reliability coefficients ranged from α = 0.65 to 0.82 across assessments and intervention groups, except for the reliability in the AgingPLUS group at the immediate posttest, which was in the lower range of acceptability (α = 0.57).
Statistical Analyses
Possible group differences between the treatment and active control groups at baseline (i.e., randomization check) were examined using independent-samples t tests for continuous variables and chi-square tests for categorical variables. Using the Proc GLM procedure in SAS 9.4 (SAS Institute Inc., 2013), analyses of covariance were performed applying an intention-to-treat (ITT) approach (White et al., 2011). We examined whether the least-squared means of changes on individual scale scores measuring participants’ NVOA, self-efficacy beliefs, and behavioral intentions from baseline differed by treatment groups, controlling for the baseline scores. As a parameter of effect size, an effect size in SD units (Cohen’s d; Cohen, 1988) for each scale score was calculated, using the quotient of the adjusted group difference divided by the overall SD of the measure at baseline.
Three sets of analyses were estimated separately for each scale score: The first set used changes observed at the Week 4 posttest (Week 4 posttest score − baseline score) as the dependent variables, the second set used changes observed at the Week 8 posttest (Week 8 posttest score − baseline score), and the third set used changes observed at the long-term posttest (Month 8 posttest score − baseline score) as the dependent variables. Group assignment was the independent variable, and the baseline score of the dependent variable was included as the covariate in all analyses.
Results
Randomization Check and Baseline Characteristics
The AgingPLUS and Health Education groups were equivalent in terms of demographic characteristics (see Table 1). Except for awareness of age-related gains and motivational self-efficacy, baseline scores on the measures of VOA, self-efficacy beliefs, and behavioral intentions were also equivalent across groups, as indicated by nonsignificant p values from t tests and chi-square tests. Means and SDs of the outcome measures at baseline by intervention group are reported in Table 2. Follow-up t tests revealed that participants in the AgingPLUS group reported higher awareness of age-related gains and higher motivational self-efficacy at baseline than active controls. This underscored the need to adjust for baseline differences when examining changes on these measures over time.
Table 2.
Descriptive Statistics (Mean and SD) for the Outcome Measures at Baseline by Intervention Group
| Variable | AgingPLUS group (n = 173) | Health Education group (n = 162) | p Value |
|---|---|---|---|
| Views of aging | |||
| Age stereotypes | 5.3 (0.9) | 5.2 (0.8) | .07 |
| Expectations regarding aging | 55.1 (15.0) | 54.4 (16.5) | .67 |
| Essentialist beliefs about aging | 17.3 (3.8) | 17.1 (4.2) | .69 |
| Awareness of age-related change gains | 19.6 (3.3) | 18.9 (3.0) | .03 |
| Awareness of age-related change losses | 11.0 (3.5) | 11.1 (3.2) | .89 |
| Self-efficacy beliefs | |||
| General self-efficacy | 27.1 (3.4) | 27.1 (3.6) | .99 |
| Self-regulation | 61.7 (28.8) | 65.7 (26.9) | .20 |
| Motivational self-efficacy | 4.6 (1.1) | 4.3 (1.2) | .03 |
| Volitional self-efficacy | 4.9 (1.0) | 4.7 (1.1) | .12 |
| Walking self-efficacy | 31.4 (5.0) | 31.1 (4.6) | .64 |
| Behavioral intentions | |||
| Exercise intention | 14.9 (2.9) | 14.8 (3.1) | .74 |
Note: SD = standard deviation.
Attrition Over Time
By the immediate posttest at Week 4, 58 participants (17.3%) had dropped out of the trial and did not provide postrandomization data (see Figure 1). This included 35 participants in the AgingPLUS group (20.2%) and 23 in the Health Education group (14.2%). By the delayed posttest at Week 8, 67 participants (20.0%) had dropped out, including 37 participants (21.4%) in the AgingPLUS group and 30 participants (18.5%) in the Health Education group. At the long-term posttest (8-month postrandomization), a total of 81 participants (24.2%) had dropped out, including 42 participants (24.3%) in the AgingPLUS group and 39 participants (24.1%) in the Health Education group. Attrition rates did not differ significantly across the two conditions, and none of the demographic variables listed in Table 1 were significant predictors of attrition over time. These attrition rates compared favorably to those reported in comparable RCTs, which can range from 25% to 50%, depending on the time frame of the follow-up assessments and the target group of participants (Linke et al., 2011).
Changes in Views of Aging
Table 3 and Figure 2, Panel A, show the covariate-adjusted change scores from baseline to immediate posttest at Week 4. As can be seen, the AgingPLUS group showed significantly greater improvements on all measures of VOA, except for awareness of age-related losses. Significant changes were found for age stereotypes, expectations regarding aging, essentialist beliefs about aging, and awareness of age-related gains (all ps < .01). For example, the AgingPLUS group showed an average improvement of 0.52 in age stereotypes, whereas the Health Education group showed a worsening of −0.03, controlling for the participants’ baseline scores (higher scores indicate more positive VOA). This was equivalent to a standardized effect size of d = 0.68, which, according to Cohen (1988), is between a medium and a large effect size (see Author Note 2).
Table 3.
Covariate-Adjusted Changes in Outcome Variables From Baseline to Immediate and Delayed Posttest by Intervention Group
| Changes from baseline to Week 4 posttest | Changes from baseline to Week 8 posttest | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | AgingPLUS group (n = 138) | Health Education group (n = 139) | d | p | AgingPLUS group (n = 136) | Health Education group (n = 132) | d | p |
| Views of aging | ||||||||
| Age stereotypes | 0.52 | −0.03 | 0.68 | <.001 | 0.58 | 0.19 | 0.48 | <.001 |
| Expectations regarding aging | 14.23 | 3.31 | 0.69 | <.001 | 12.46 | 1.83 | 0.67 | <.001 |
| Essentialist beliefs about aging | 2.50 | 0.66 | 0.48 | <.001 | 3.02 | −0.08 | 0.80 | <.001 |
| Awareness of age-related gains | 2.09 | 1.31 | 0.25 | .003 | 1.99 | 1.07 | 0.29 | .001 |
| Awareness of age-related losses | −0.66 | −0.13 | −0.16 | .06 | −1.22 | −0.46 | −0.23 | .016 |
| Self-efficacy beliefs | ||||||||
| General self-efficacy | 1.42 | 0.51 | 0.27 | <.001 | 1.44 | 0.26 | 0.36 | .006 |
| Self-regulation scale | 0.62 | 0.58 | 0.01 | .91 | 1.83 | 1.27 | 0.12 | .14 |
| Motivational self-efficacy | 0.74 | 0.23 | 0.45 | <.001 | 0.77 | 0.20 | 0.50 | <.001 |
| Volitional self-efficacy | 0.60 | 0.16 | 0.43 | <.001 | 0.69 | 0.03 | 0.64 | <.001 |
| Walking self-efficacy | 9.56 | 8.73 | 0.03 | .71 | 12.27 | 11.39 | 0.03 | .69 |
| Behavioral intentions | ||||||||
| Exercise intention | 2.00 | 0.65 | 0.47 | <.001 | 1.84 | 0.72 | 0.39 | <.001 |
Figure 2.
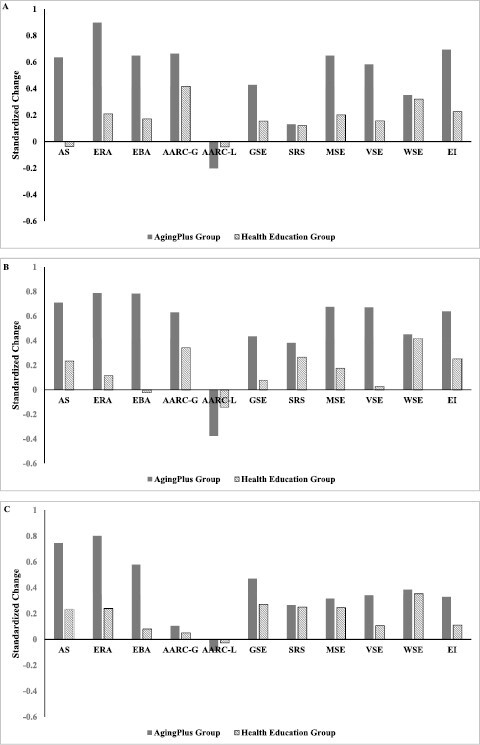
Covariate-adjusted changes in views of aging, self-efficacy beliefs, and behavioral intentions in the AgingPLUS and the Health Education group at immediate posttest (Panel A), delayed posttest (Panel B), and long-term posttest (Panel C).
Improvements in participants’ expectations regarding aging were found in both groups, but the improvements were significantly greater in the AgingPLUS group. The standardized effect size was d = 0.69, indicating a medium-to-large effect. Similarly, participants’ essentialist beliefs about aging became more positive from baseline to the immediate posttest, and the improvement was again significantly stronger in the AgingPLUS group compared to the control group. The effect size was d = 0.48, indicating a medium effect of the intervention.
Finally, changes from baseline to immediate posttest were also in the positive direction for participants’ awareness of age-related gains. Participants in the AgingPLUS group improved significantly more than participants in the Health Education group. The effect size was d = 0.25, indicating a small, standardized effect. No significant differences in changes from baseline to immediate posttest were found between the treatment and control group in terms of participants’ awareness of age-related losses.
Table 3 and Figure 2, Panel B, show that the positive changes in participants’ VOA persisted in the AgingPLUS group to the delayed posttest at Week 8. Again, participants in the AgingPLUS group showed improvements in age stereotypes, expectations regarding aging, essentialist beliefs of aging, and awareness of age-related gains that were significantly larger than the changes in the Health Education group. Standardized effect sizes for the statistically significant effects ranged from d = −0.23 for awareness of age-related losses to d = 0.80 for essentialist beliefs about aging. In contrast to Week 4, participants in the AgingPLUS group now also showed significantly larger changes in terms of awareness of age-related losses than those in the Health Education group.
Table 4 and Figure 2, Panel C, show that the positive changes in participants’ VOA lasted to the long-term posttest, which was 8-month postrandomization. Again, participants in the AgingPLUS group showed significant improvements in age stereotypes, expectations regarding aging, essentialist beliefs of aging, and awareness of age-related gains compared to baseline and compared to the changes in the Health Education group. Additionally, participants in the AgingPLUS group showed at this assessment also significantly lower awareness of age-related losses (i.e., fewer loss-related experiences) compared to baseline and compared to participants in the Health Education group. Standardized effect sizes for the significant effects ranged from d = 0.05 for awareness of age-related gains to d = 0.56 for expectations regarding aging, indicating small- to medium-sized effects at the long-term follow-up.
Table 4.
Covariate-Adjusted Changes in Outcome Variables From Baseline to Long-Term Posttest (Month 8) by Intervention Group
| Variable | AgingPLUS group (n = 131) | Health Education group (n = 123) | d | p |
|---|---|---|---|---|
| Views of aging | ||||
| Age stereotypes | 0.61 | 0.19 | 0.51 | <.001 |
| Expectations regarding aging | 12.70 | 3.79 | 0.56 | <.001 |
| Essentialist beliefs about aging | 2.23 | 0.31 | 0.50 | <.001 |
| Awareness of age-related gains | 0.33 | 0.16 | 0.05 | .006 |
| Awareness of age-related losses | −0.29 | −0.09 | −0.06 | .001 |
| Self-efficacy beliefs | ||||
| General self-efficacy | 1.56 | 0.90 | 0.20 | .036 |
| Self-regulation scale | 1.27 | 1.20 | 0.01 | .868 |
| Motivational self-efficacy | 0.36 | 0.28 | 0.07 | .530 |
| Volitional self-efficacy | 0.35 | 0.11 | 0.23 | .033 |
| Walking self-efficacy | 10.46 | 9.61 | 0.03 | .735 |
| Behavioral intentions | ||||
| Exercise intention | 0.95 | 0.32 | 0.22 | .075 |
Changes in Self-Efficacy Beliefs
Table 3 and Figure 2, Panel A, also show the covariate-adjusted changes from baseline to the immediate posttest (Week 4) for participants’ self-efficacy beliefs. Again, the AgingPLUS group had significantly greater improvements on general perceived self-efficacy, motivational self-efficacy, and volitional self-efficacy compared to the Health Education group (all ps < .001). Effect sizes ranged from d = 0.27 for general perceived self-efficacy to d = 0.45 for motivational self-efficacy, indicating small-to-medium effects of the intervention. No significant intervention effects were found for the self-regulation scale and participants’ walking self-efficacy.
Table 3 and Figure 2, Panel B, show that the intervention effects for general perceived self-efficacy, motivational self-efficacy, and volitional self-efficacy were maintained through the delayed posttest at Week 8. Indeed, the effect sizes improved slightly and now ranged from d = 0.36 for general perceived self-efficacy to d = 0.64 for volitional self-efficacy. Again, no significant intervention effects were found for the self-regulation scale and walking self-efficacy.
Table 4 and Figure 2, Panel C, show that the intervention effects for general self-efficacy (d = 0.20) and volitional self-efficacy (d = 0.23) persisted to the long-term posttest, whereas the previously significant effect for motivational self-efficacy became nonsignificant. The findings also indicated that there was a decay in the intervention effect as the effect sizes for the significant effects dropped from medium to small (see Figure 3).
Figure 3.
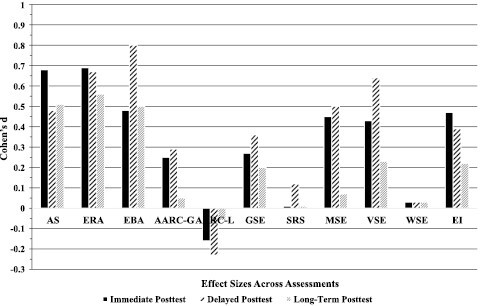
Effect sizes (Cohen’s d) across the three times of intervention assessment.
Changes in Behavioral Intentions
Table 3 and Figure 2, Panel A, show the covariate-adjusted changes in participants’ exercise intention from baseline to the immediate posttest at Week 4. The AgingPLUS group showed a significantly greater improvement in terms of exercise intention, and this effect was maintained to the delayed posttest at Week 8 (both ps < .001; see Table 3 and Figure 2, Panel B). At Week 4, the AgingPLUS group showed an average increase of 2.00 points in exercise intention (1.84 points at Week 8), whereas the Health Education group experienced an increase of 0.65 points (0.72 points at Week 8), controlling for baseline scores. The effect sizes were d = 0.47 at Week 4 and d = 0.39 at Week 8, indicating medium effects.
Table 4 and Figure 2, Panel C, show that the covariate-adjusted changes in participants’ exercise intention from baseline to the 8-month postrandomization follow-up were no longer statistically significant, as they had been at the previous posttests.
Discussion
Findings from this RCT showed that the 4-week intervention significantly improved middle-aged and older adults’ NVOA, self-efficacy beliefs, and behavioral intentions—a tripartite cluster of social-cognitive and motivational variables considered critical for successful behavior change (Nielsen & Reiss, 2012). In terms of NVOA, compared to the control group, adults who participated in the AgingPLUS program showed significantly greater positive changes in age stereotypes, expectations regarding aging, essentialist beliefs about aging, and a greater change in awareness of age-related gains at the immediate and delayed posttest and at the long-term posttest 8-month postrandomization. In addition, at the long-term posttest, participants in the AgingPLUS program also reported significantly fewer loss-related aging experiences compared to baseline and compared to the participants in the control group. Thus, the AgingPLUS program was effective in making participants’ NVOA consistently more positive and strengthened their positive VOA (e.g., awareness of age-related gains). Most notably, the effects were stronger for individuals’ general VOA, such as negative age stereotypes, expectations regarding aging, and essentialist beliefs about aging compared to their personal VOA (i.e., awareness of age-related gains and losses).
Similar findings were obtained for most of the measures of self-efficacy and the indicator of behavioral intentions. Specifically, participants in the AgingPLUS group showed significant positive changes from baseline to immediate and delayed posttests and compared to the control group in their general perceived self-efficacy, motivational self-efficacy, and volitional self-efficacy. These effects were maintained to the 8-month postrandomization follow-up for general self-efficacy and volitional self-efficacy. No significant changes were observed in terms of participants’ self-regulation ability and walking self-efficacy. In terms of behavioral intentions, both at the immediate and the delayed posttest, participants in the AgingPLUS group reported significantly greater changes in their exercise intention than the participants in the Health Education control group. This effect, however, became nonsignificant at the long-term posttest.
In summary, findings from this intervention trial showed that middle-aged and older adults’ NVOA, self-efficacy beliefs, and exercise intentions were malleable and were made more positive via the AgingPLUS intervention program. Similar effects were not observed in the Health Education control group. Moreover, most of the obtained effects stayed significant up to 8 months after randomization. Yet, effect sizes were smaller at the long-term posttest, suggesting that the intervention effects in the AgingPLUS group showed some weakening over time (see Figure 3). Overall, the obtained effect sizes were in the same range or larger than reported in related meta-analyses (Sheeran et al., 2016; Webb & Sheeran, 2006; Zhang et al., 2019).
Contribution to the Literature
Although previous studies have provided evidence of the malleability of select social-cognitive variables that may underlie adults’ negative attitudes and misconceptions toward engaging in physical activity, the present study is the first one to examine the malleability of a tripartite cluster of variables, namely general and personal NVOA, self-efficacy beliefs, and behavioral intentions, using the experimental medicine approach (Riddle, 2015). We discuss the relevance of the findings regarding each cluster component individually to underscore the study’s specific contributions to the literature.
In terms of adults’ NVOA it is often assumed that they are immutable because they develop from early on in individuals’ lives (Levy, 2009). Although this skepticism seems justified, several previous studies did show that both implicit and explicit age stereotypes (Levy et al., 2014; Nehrkorn-Bailey et al., 2023) can be altered and that observed changes were associated with important behavioral outcomes. The major contribution of the present study to the ongoing debate is threefold. First, whereas the previous studies were based on smaller samples, the present study was a properly powered RCT, resulting in more reliable and valid estimates of the intervention effects on measures of NVOA.
Second, the present study contributes to the literature by showing that the effects of the AgingPLUS intervention were not limited to a single NVOA measure but generalized across measures, including measures of general and personal VOA. Indeed, it is noteworthy that the obtained effect sizes were consistently larger for measures of general NVOA compared to the measures of personal NVOA. This was an unexpected finding given that general NVOA, because of their cultural and societal roots (Levy, 2009), are assumed to be less malleable compared to personal NVOA. At the same time, it is not entirely surprising that personal NVOA showed less malleability in this trial. Because personal NVOA are rooted in individuals’ actual experiences (i.e., real-life perceptions and sensations), they may be harder to refute through a psychoeducational intervention than the negative stereotypes that exist in the surrounding culture. However, the delayed significant positive change in participants’ perceptions of aging-related losses at the long-term posttest may also suggest that personal NVOA can only be overridden after longer-lasting personal experiences. This assumption, however, will require further examination (for a similar example in adult personality research, see Mühlig-Versen et al., 2012).
Third, the present study is the first one showing that the intervention effects regarding adults’ NVOA persisted beyond the immediate and the delayed posttest up to 8 months after postrandomization. Although the effect sizes showed some decline from the Week 8 posttest to the long-term posttest, the positive changes compared to baseline and compared to the control group stayed significant, documenting for the first time that NVOA can be changed in a durable way with a rather brief intervention.
In terms of participants’ self-efficacy beliefs and behavioral intentions, the contribution of the present study consists in the documentation of comparable or larger effect sizes as reported in previous meta-analyses (Sheeran et al., 2016; Webb & Sheeran, 2006; Zhang et al., 2019). This suggests that the AgingPLUS program may be a viable program to improve adults’ self-efficacy beliefs as a basic mechanism of behavior change. Interestingly, the effects were largest for participants’ motivational and volitional self-efficacy beliefs (ds ranging from 0.43 to 0.64) and even improved from the immediate to the delayed posttest. This finding suggests that the AgingPLUS program strengthened participants’ self-efficacy beliefs both in the motivational, preintentional phase as well as in the volitional, postintentional phase of the intervention (Schwarzer, 2008), which may overall translate into greater intervention effects on the behavioral outcomes. Similarly, the AgingPLUS program improved participants’ exercise intention both at the immediate and the delayed posttest, but this effect became nonsignificant at the long-term posttest. The effect sizes for this putative mechanism of the intervention program were in the same range as reported in the meta-analysis by Webb and Sheeran (2006).
Strengths and Limitations of the Current Study
The current study has several noteworthy strengths. First, the RCT was theory-based and included a carefully designed active control group (Newman et al., 2010). Given that all the observed changes were significantly larger in the AgingPLUS group compared to the control group, this permits the conclusion that the effects were specific to the content of the intervention, a necessary requirement of the experimental medicine approach (Riddle, 2015; Sheeran et al., 2016). Second, the RCT was conducted with trained and certified facilitators and testers who were blind to the trial conditions, ensuring that no facilitator or tester bias was introduced into the trial. Furthermore, the application of rigorous quality control procedures (e.g., video review of intervention sessions; continuous monitoring of testers) ensured high intervention and assessment fidelity. Third, careful assessment implementation with trained and certified testers ensured that the amount of missing data was kept at a minimum. Fourth, implementation of the ITT approach provides conservative estimates of the intervention effects on the putative mechanisms of treatment efficacy, avoiding too liberal evaluations of the intervention’s efficacy. Fifth, we consider it a strength that the effects of the AgingPLUS program were seen across a tripartite cluster of social-cognitive and motivational variables that target mechanistic pathways (Riddle, 2015) for increasing physical activity in sedentary adults. Finally, the findings are also promising in that they show both immediate and lasting effects of the AgingPLUS intervention.
Despite these strengths, several limitations need to be acknowledged. First, the study focused explicitly on adults between the age of 45 and 75, which limits the generalizability of the findings to adults outside this age range. Second, the study sample was relatively homogeneous in terms of gender, race/ethnicity, and level of education, limiting the generalizability of findings to non-White adults, to adults with lower education, and to men. Future adaptions of the program need to focus on recruiting adults from more diverse backgrounds and traditionally underrepresented groups of middle-aged and older adults. Future work should also assess the effect of modifications to the design of the program that could improve outcomes (e.g., culturally tailoring the content) and improve access for the broader population (e.g., web-based or in-home computerized training). Third, because participation in the RCT required in-person attendance of the group meetings, this requirement may have limited the access to middle-aged and older adults in rural settings farther away from the intervention site and to adults with limited transportation or mobility problems. Fourth, the reliabilities for three outcome measures (i.e., essentialist beliefs about aging; awareness of age-related gains; behavioral intentions) at the immediate posttest were in the lower range of acceptability. Although all measures had been selected because of their well-established psychometric characteristics, the lower reliabilities at this occasion of assessment very likely resulted from a change in administration format and do not necessarily render the measures invalid. That is, compared to the other assessments, which were performed in one-on-one sessions, this specific assessment was conducted in a group setting at the end of the last group meeting. This format may have inadvertently affected the way the participants responded to the questions. Finally, although major efforts were made to minimize the effects of the COVID-19 pandemic on the RCT, the pandemic caused a major interruption and subsequent participant enrollment proceeded at a slower pace and with a lower completion rate.
In summary, the present study contributes to the literature on multifactorial interventions by showing that participation in the AgingPLUS program significantly moved the measures of a cluster of putative social-cognitive and motivational mechanisms. Focusing on the malleability and dynamic interplay of these mechanisms may lead to broader health benefits rather than focusing on individual mechanisms alone and may inform future work in intervention research. Moreover, following the experimental medicine approach helps to gain a better understanding of the relative importance of different mechanistic pathways in behavioral interventions (Riddle, 2015).
Supplementary Material
Contributor Information
Manfred Diehl, Department of Human Development and Family Studies, Colorado State University, Fort Collins, Colorado, USA.
George W Rebok, Department of Mental Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA; Center on Aging and Health, School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA.
David L Roth, Center on Aging and Health, School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA.
Abigail Nehrkorn-Bailey, Department of Psychology, University of Wisconsin–Green Bay, Green Bay, Wisconsin, USA.
Diana Rodriguez, Department of Human Development and Family Studies, Colorado State University, Fort Collins, Colorado, USA.
Han-Yun Tseng, Department of Human Development and Family Studies, Colorado State University, Fort Collins, Colorado, USA.
Diefei Chen, Center on Aging and Health, School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA.
Author Notes
1. Although the constructs of personal control and self-efficacy address slightly different aspects of human behavior, they are theoretically very close (Robinson & Lachman, 2017). First, both concepts refer to individuals’ beliefs. Second, personal control beliefs capture persons’ beliefs whether they can exert control over certain aspects of their lives (e.g., health or well-being). In comparison, self-efficacy beliefs refer to individuals’ confidence that they have the skills to achieve valued outcomes in certain behavioral domains. Thus, both concepts refer to individuals’ sense of control over their environment and behavior (Bandura, 1997). In research practice, measures of perceived control and self-efficacy are often used interchangeably.
2. Additional analyses not reported here showed that significant positive changes in age stereotypes were found for all subscales of the Age Stereotypes Scale. Effect size coefficients generally indicated medium effect sizes. The same was the case for the three subscales of the Expectations Regarding Aging scale.
Funding
The AgingPLUS clinical trial was funded by the National Institute on Aging, National Institutes of Health (R01 AG051723; Principal Investigator: M. Diehl).
Conflict of Interest
None.
Data Availability
The deidentified data of the AgingPLUS trial are available from the principal investigator upon request for replication purposes. The study reported in this article was not preregistered.
References
- Ashford, S., Edmunds, J., & French, D. P. (2010). What is the best way to change self-efficacy to promote lifestyle and recreational physical activity? A systematic review with meta-analysis. British Journal of Health Psychology, 15(2), 265–288. 10.1348/135910709X461752 [DOI] [PubMed] [Google Scholar]
- Bandura, A. (1997). Self-efficacy: The exercise of control. Freeman. [Google Scholar]
- Beyer, A. K., Wolff, J. K., Freiberger, E., & Wurm, S. (2019). Are self-perceptions of ageing modifiable? Examination of an exercise programme with vs. without a self-perceptions of ageing-intervention for older adults. Psychology & Health, 34(6), 661–676. 10.1080/08870446.2018.1556273 [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Erlbaum. [Google Scholar]
- Diehl, M., Brothers, A., & Wahl, H.-W. (2021). Self-perceptions and awareness of aging: Past, present, and future. In Schaie K. W. & Willis S. L. (Eds.), Handbook of the psychology of aging (9th ed., pp. 155–179). Academic Press. 10.1016/B978-0-12-816094-7.00001-5 [DOI] [Google Scholar]
- Diehl, M., Nehrkorn-Bailey, A., Thompson, K., Rodriguez, D., Li, K., Rebok, G. W., Roth, D. L., Chung, S.-E., Bland, C., Feltner, S., Forsyth, G., Hulett, N., Klein, B., Mars, P., Martinez, K., Mast, S., Monasterio, R., Moore, K., Schoenberg, H., ... Tseng, H. Y. (2020). The AgingPLUS trial: Design of a randomized controlled trial to increase physical activity in middle-aged and older adults. Contemporary Clinical Trials, 96, 106105. 10.1016/j.cct.2020.106105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl, M., Semegon, A. B., & Schwarzer, R. (2006). Assessing attention control in goal pursuit: A component of dispositional self-regulation. Journal of Personality Assessment, 86(3), 306–317. 10.1207/s15327752jpa8603_06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French, D. P., Olander, E. K., Chisholm, A., & McSharry, J. (2014). Which behaviour change techniques are most effective at increasing older adults’ self-efficacy and physical activity behaviour? A systematic review. Annals of Behavioral Medicine, 48, 225–234. 10.1007/s12160-014-9593-z [DOI] [PubMed] [Google Scholar]
- Guralnik, J. M., Simonsick, E. M., Ferrucci, L., Glynn, R. J., Berkman, L. F., Blazer, D. G., & Wallace, R. B. (1994). A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology, 49(2), M85–M94. 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- Kaspar, R., Gabrian, M., Brothers, A., Wahl, H.-W., & Diehl, M. (2019). Measuring awareness of age-related change: Development of a 10-item short form for use in large-scale surveys. Gerontologist, 59(3), e130–e140. 10.1093/geront/gnx213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornadt, A. E., & Rothermund, K. (2011). Contexts of aging: Assessing evaluative age stereotypes in different life domains. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66(5), 547–556. 10.1093/geronb/gbr036 [DOI] [PubMed] [Google Scholar]
- Krause, N. (2007). Age and decline in role-specific feelings of control. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 62(1), S28–S35. 10.1093/geronb/62.1.s28 [DOI] [PubMed] [Google Scholar]
- Lachman, M. E., & Agrigoroaei, S. (2010). Promoting functional health in midlife and old age: Long-term protective effects of control beliefs, social support, and physical exercise. PLoS One, 5(10), e13297. 10.1371/journal.pone.0013297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman, M. E., Andreoletti, C., & Pearman, A. (2006). Memory control beliefs: How are they related to age, strategy use and memory improvement? Social Cognition, 24(3), 359–385. 10.1521/soco.2006.24.3.359 [DOI] [Google Scholar]
- Lachman, M. E., Lipsitz, L., Lubben, J., Castaneda-Sceppa, C., & Jette, A. M. (2018). When adults don’t exercise: Behavioral strategies to increase physical activity in sedentary middle-aged and older adults. Innovation in Aging, 2(1), 1–12. 10.1093/geroni/igy007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman, M. E., Neupert, S. D., & Agrigoroaei, S. (2011). The relevance of control beliefs for health and aging. In Schaie K. W. & Willis S. L. (Eds.), Handbook of the psychology of aging (7th ed., pp. 175–190). Academic Press. 10.1016/B978-0-12-380882-0.00011-5 [DOI] [Google Scholar]
- Levy, B. (1996). Improving memory in old age through implicit self-stereotyping. Journal of Personality and Social Psychology, 71(6), 1092–1107. 10.1037//0022-3514.71.6.1092 [DOI] [PubMed] [Google Scholar]
- Levy, B. R. (2009). Stereotype embodiment: A psychosocial approach to aging. Current Directions in Psychological Science, 18, 332–336. 10.1111/j.1467-8721.2009.01662.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, B. R., & Myers, L. M. (2004). Preventive health behaviors influenced by self-perceptions of aging. Preventive Medicine, 39(3), 625–629. 10.1016/j.ypmed.2004.02.029 [DOI] [PubMed] [Google Scholar]
- Levy, B. R., Pilver, C., Chung, P. H., & Slade, M. D. (2014). Subliminal strengthening: Improving older individuals’ physical function over time with an implicit-age stereotype intervention. Psychological Science, 25(12), 2127–2135. 10.1177/0956797614551970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, B. R., Slade, M. D., Kunkel, S. R., & Kasl, S. V. (2002). Longevity increased by positive self-perceptions of aging. Journal of Personality and Social Psychology, 83(2), 261–270. 10.1037//0022-3514.83.2.261 [DOI] [PubMed] [Google Scholar]
- Lindland, E., Kendall-Taylor, N., Haydon, A., & Fond, M. (2016). Gauging aging: Expert and public understandings of aging in America. Communication and the Public, 1(2), 211–229. 10.1177/2057047315625340 [DOI] [Google Scholar]
- Linke, S. E., Gallo, L. C., & Norman, G. J. (2011). Attrition and adherence rates of sustained vs. intermittent exercise interventions. Annals of Behavioral Medicine: Annals of Behavioral Medicine, 42(2), 197–209. 10.1007/s12160-011-9279-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J., Rosas, L. G., & Lv, N. (2016). Precision lifestyle medicine: A new frontier in the science of behavior change and population health. American Journal of Preventive Medicine, 50(3), 395–397. 10.1016/j.amepre.2015.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas, C. J. M., & Hox, J. J. (2005). Sufficient sample sizes for multilevel modeling. Methodology, 1(1), 85–91. 10.1027/1614-2241.1.3.85 [DOI] [Google Scholar]
- McAuley, E., Blissmer, B., Katula, J., & Duncan, T. E. (2000). Exercise environment, self-efficacy, and affective responses to acute exercise in older adults. Psychology & Health, 15(3), 341–355. 10.1080/08870440008401997 [DOI] [Google Scholar]
- Moher, D., Hopewell, S., Schulz, K. F., Montori, V., Gøtzsche, P. C., Devereaux, P. J., Elbourne, D., Egger, M., Altman, D. G., & the Consolidated Standards of Reporting Trials Group. (2010). CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology, 63(8), e1–e37. 10.1016/j.jclinepi.2010.03.004 [DOI] [PubMed] [Google Scholar]
- Mühlig-Versen, A., Bowen, C. E., & Staudinger, U. M. (2012). Personality plasticity in later adulthood: Contextual and personal resources are needed to increase openness to experience. Psychology and Aging, 27(4), 855–866. 10.1037/a0029357 [DOI] [PubMed] [Google Scholar]
- Nehrkorn-Bailey, A. M., Rodriguez, D., Forsyth, G., Braun, B., Burke, K., & Diehl, M. (2023). Change in views of aging, physical activity, and physical health over 8 weeks: Results from a randomized study. Journal of Aging and Physical Activity. Advance online publication. 10.1123/japa.2022-0133 [DOI] [PMC free article] [PubMed]
- Newman, A. B., Bayles, C. M., Milas, C. N., McTigue, K., Williams, K., Robare, J. F., Taylor, C. A., Albert, S. M., & Kuller, L. H. (2010). The 10 Keys to Healthy Aging: Findings from an innovative prevention program in the community. Journal of Aging and Health, 22(5), 547–566. 10.1177/0898264310363772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, L., & Reiss, D. (2012). Motivation and aging: Toward the next generation of behavioral interventions.https://www.nia.nih.gov/sitesdefault/files/d7/background.pdf
- Parisi, J. M., Gross, A. L., Marsiske, M., Willis, S. L., & Rebok, G. W. (2017). Control beliefs and cognition over a 10-year period: Findings from the ACTIVE trial. Psychology and Aging, 32(1), 69–75. 10.1037/pag0000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich, A., Kellar, I., Parker, R., MacRae, S., Learmonth, M., Sykes, B., Taylor, N., & Castle, H. (2014). How can self-efficacy be increased? Meta-analysis of dietary interventions. Health Psychology Review, 8(3), 270–285. 10.1080/17437199.2013.813729 [DOI] [PubMed] [Google Scholar]
- Riddle, M. (2015). News from the NIH: Using an experimental medicine approach to facilitate translational research. Translational Behavioral Medicine, 5(4), 486–488. 10.1007/s13142-015-0333-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, S. A., & Lachman, M. E. (2017). Perceived control and aging: A mini-review and directions for future research. Gerontology, 63(5), 435–442. 10.1159/000468540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent-Cox, K. A., Anstey, K. J., & Luszcz, M. A. (2012). The relationship between change in self-perceptions of aging and physical functioning in older adults. Psychology and Aging, 27(3), 750–760. 10.1037/a0027578 [DOI] [PubMed] [Google Scholar]
- Sarkisian, C. A., Prohaska, T. R., Davis, C., & Weiner, B. (2007). Pilot test of an attribution retraining intervention to raise walking levels in sedentary older adults. Journal of the American Geriatrics Society, 55(11), 1842–1846. 10.1111/j.1532-5415.2007.01427.x [DOI] [PubMed] [Google Scholar]
- Sarkisian, C. A., Steers, W. N., Hays, R. D., & Mangione, C. M. (.2005). Development of the 12-item Expectations Regarding Aging Survey. Gerontologist, 45(2), 240–248. 10.1093/geront/45.2.240 [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. (2013). SAS/STAT 9.4 user’s guide. SAS Institute. [Google Scholar]
- Schwarzer, R. (2008). Modeling health behavior change: How to predict and modify the adoption and maintenance of health behaviors. Applied Psychology, 57(1), 1–29. 10.1111/j.1464-0597.2007.00325.x [DOI] [Google Scholar]
- Schwarzer, R., & Jerusalem, M. (1995). Generalized self-efficacy scale. In Weinman J., Wright S., & Johnston M. (Eds.), Measures in health psychology: A user’s portfolio (pp. 35–37). GL Assessment. [Google Scholar]
- Sheeran, P., Maki, A., Montanaro, E., Avishai-Yitshak, A., Bryan, A., Klein, W. M. P., Miles, E., & Rothman, A. J. (2016). The impact of changing attitudes, norms, and self-efficacy on health-related intentions and behavior: A meta-analysis. Health Psychology, 35(11), 1178–1188. 10.1037/hea0000387 [DOI] [PubMed] [Google Scholar]
- Sheeran, P., & Webb, T. L. (2016). The intention-behavior gap. Social and Personality Psychology Compass, 10(9), 503–518. 10.1111/spc3.12265 [DOI] [Google Scholar]
- Siebert, J. S., Wahl, H.-W., & Schröder, J. (2018a). The role of attitude toward own aging for fluid and crystallized functioning: 12-year evidence from the ILSE study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73(5), 836–845. 10.1093/geronb/gbw050. [DOI] [PubMed] [Google Scholar]
- Webb, T. L., & Sheeran, P. (2006). Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychological Bulletin, 132(2), 249–268. 10.1037/0033-2909.132.2.249 [DOI] [PubMed] [Google Scholar]
- Weiss, D., & Diehl, M. (2021). Measuring (non)essentialist beliefs about the process of aging. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 76(7), 1340–1348. 10.1093/geronb/gbaa113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, R. L., Bagwell, D. K., & Dark-Freudeman, A. (2008). Self-efficacy and memory aging: The impact of a memory intervention based on self-efficacy. Aging, Neuropsychology, and Cognition, Neuropsychology and Cognition, 15(3), 302–329. 10.1080/13825580701440510 [DOI] [PubMed] [Google Scholar]
- White, I. R., Horton, N. J., Carpenter, J., & Pocock, S. J. (2011). Strategy for intention to treat analysis in randomized trials with missing outcome data. BMJ, 342, d40. 10.1136/bmj.d40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff, J. K., Warner, L. M., Ziegelmann, J. P., & Wurm, S. (2014). What do targeting positive views on ageing add to a physical activity intervention in older adults? Results from a randomised controlled trial. Psychology & Health, 29(8), 915–932. 10.1080/08870446.2014.896464 [DOI] [PubMed] [Google Scholar]
- Wurm, S., & Schäfer, S. K. (2022). Gain- but not loss-related self-perceptions of aging predict mortality over a period of 23 years: A multidimensional approach. Journal of Personality and Social Psychology, 123(3), 636–653. 10.1037/pspp0000412 [DOI] [PubMed] [Google Scholar]
- Zhang, C.-Q., Zhang, R., Schwarzer, R., & Hagger, M. S. (2019). A meta-analysis of the health action approach. Health Psychology, 38(7), 623–637. 10.1037/hea0000728 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The deidentified data of the AgingPLUS trial are available from the principal investigator upon request for replication purposes. The study reported in this article was not preregistered.


