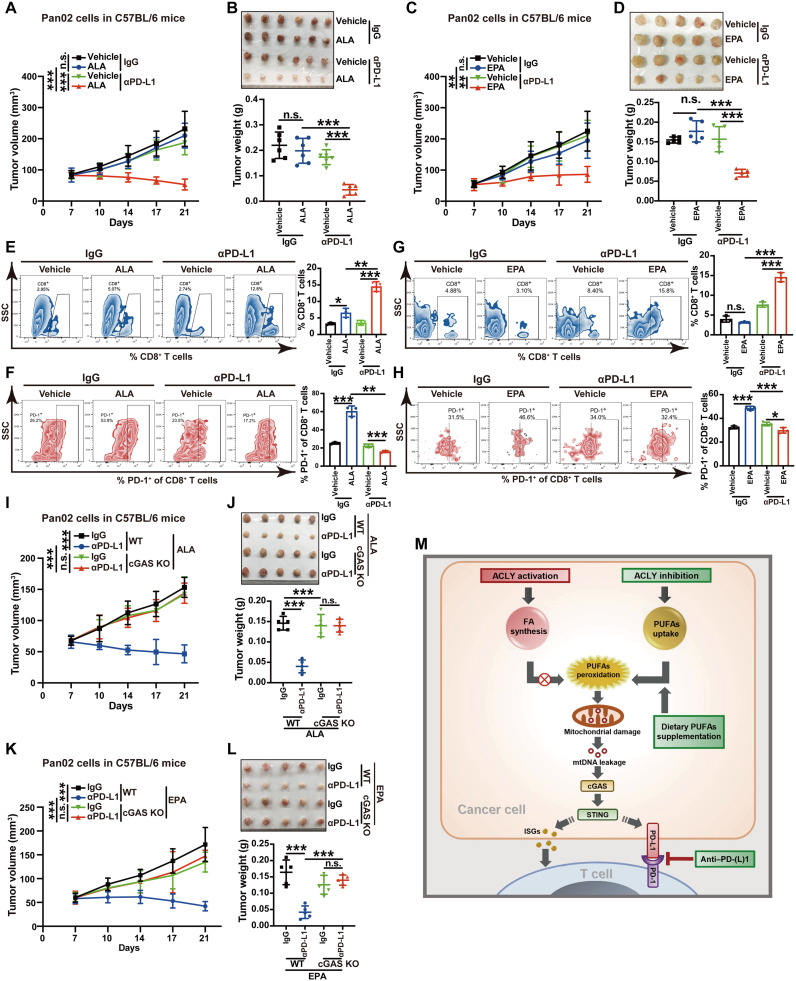
Fig. 7. Dietary PUFA supplementation mirrors the enhanced efficacy of PD-L1 blockade by ACLY inhibition.
(A and B) Tumor growth (A) and tumor burdens (B) in C57BL/6 mice injected subcutaneously with Pan02 cells with the treatment of ALA and αPD-L1 antibodies either alone or in combination. (C and D) Tumor growth (C) and tumor burdens (D) in C57BL/6 mice injected subcutaneously with Pan02 cells with treatment of EPA and αPD-L1 antibodies either alone or in combination. (E and F) Flow staining and frequency of indicated cells in indicated tumors. (G and H) Flow staining and frequency of indicated cells in indicated tumors. (I and J) Tumor growth (I) and tumor burdens (J) in C57BL/6 mice injected subcutaneously with Pan02 cells with cGAS KO or not with the treatment of ALA and αPD-L1 antibodies either alone or in combination. (K and L) Tumor growth (K) and tumor burdens (L) in C57BL/6 mice injected subcutaneously with Pan02 cells with cGAS KO or not with treatment of EPA and αPD-L1 antibodies either alone or in combination. (M) Summary scheme. ACLY activation sustains higher de novo fatty acid (FA) synthesis to prevent excessive PUFA uptake, while ACLY inhibition reduces de novo FA synthesis but enhances the uptake of excessive PUFAs, which induces mitochondrial damage via lipid peroxidation, further triggering mtDNA leakage to activate the cGAS-STING pathway and lastly boosting PD-(L)1 blockade efficacy. n = 6 mice per group from one independent experiment [(A) and (B)]; n = 5 mice per group from one independent experiment [(C), (D), and (I) to (L)]; n = 3 biological replicates from one independent experiment [(F), (G), and (M) to (O)]. Statistical significance was assessed by two-way ANOVA [(A), (C), (I), and (K)] and one-way ANOVA [(B), (D) to (H), (J), and (L)]; *P < 0.05; **P < 0.01; ***P < 0.001.