Figure 4.
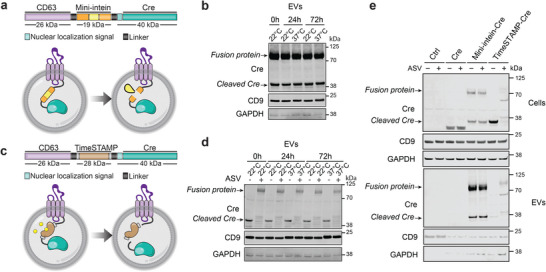
Protein loading of genetically engineered EVs. a) Schematic representation of the design of the DnaB mini‐intein fusion protein construct and the EV loading system. The tetraspanin CD63 (violet) was fused with a DnaB mini‐intein cassette consisting of a mini‐intein protein domain (yellow) and flanking extein (orange). The C‐term of the mini‐intein cassette was connected with Cre recombinase (green) with an N‐terminal nuclear localization sequence (light green) (NLS‐Cre). NLS‐Cre recombinase is then recruited in the EV lumen during biogenesis as a fusion with CD63. DnaB mini‐intein consists of the splicing domain and is modified to catalyze cleavage at its termini. Mini‐intein‐mediated cleavage reaction releases Cre recombinase from CD63‐Cre fusion protein. b) Representative western blot analyses of the Cre recombinase in EVs loaded with the DnaB mini‐intein system as a function of time and temperature. EVs isolated from Expi293F cells transfected with the mini‐intein loading system were incubated for 0, 24, or 72 h at 22 or 37 °C. GAPDH was used as a loading control and CD9 as an EV marker. c) Schematic representation of the design of a protein loading system with a TimeSTAMP (Time‐Specific Tagging for the Age Measurement of Proteins) drug‐controllable cargo release and loading in EVs. As above, between CD63 (violet) and NLS‐Cre recombinase (green) TimeSTAMP protein (brown) was inserted. The epitope tag (brown) is rapidly removed from the protein of interest by a sequence‐specific protease unless a protease inhibitor Asunaprevir (ASV) is present (shown in yellow). This approach allows the release of Cre recombinase from the fusion with CD63 protein in the EV lumen, once ASV is absent. d) Representative western blot analyses of the Cre recombinase EVs as a function of time and temperature. EVs were isolated from Expi293F cells transfected with the TimeSTAMP loading system. Cells were cultured with and without ASV (3 µm). EVs were incubated for 0, 24, or 72 h at 22 or 37 °C. GAPDH was used as a loading control and CD9 as an EV marker. e) Representative western blot analyses of cells and EVs secreted by Expi293F cells expressing the DnaB mini‐intein or TimeSTAMP loading systems. Cells were transfected with mini‐intein and TimeSTAMP constructs, plasmid overexpressing Cre recombinase or mock (Ctrl sample), and cultured with 3 M of ASV in indicated conditions. Cell supernatant was collected 48 h after transfection and subjected to differential centrifugation. Both loading approaches successfully brought cargo protein inside EVs. Levels of free Cre recombinase inside EVs can be estimated by the intensity of Cre protein bands ≈38 kDa. Bands ≈70 kDa correspond to CD63‐Cre fusion protein. GAPDH was used as a loading control and CD9 as an EV marker.
