Figure 9.
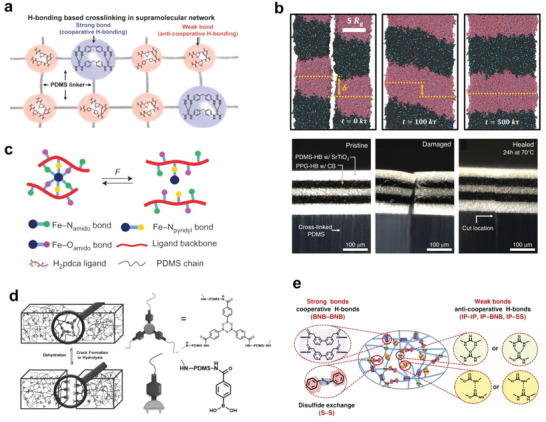
Arrangements of diverse dynamic bonds in PDMS. a–c) Dynamic non‐covalent bonds: a) combinations of strong and weak hydrogen bonds, with the strong transferring elasticity and the weak dissipating energy; reproduced with permission.[ 129 ] Copyright 2018, Wiley‐VCH. b) multilayered polymeric films undergo autonomous realignment upon damage to minimize interfacial free energy during the healing process, with simulation (top) and microscopic observation (bottom); reproduced with permission.[ 131 ] Copyright 2023, American Association for the Advancement of Science. c) Fe(III)‐N bonds for modulus enhancement and Fe(III)‐O bonds for energy dissipation, both stabilized by chelation. Reproduced with permission.[ 137 ] Copyright 2016, Nature Publishing Group. d) Dynamic covalent bonds: reversible equilibrium transition of boroxane bonds in response to humidity stimulation. Reproduced with permission.[ 146 ] Copyright 2016, Wiley‐VCH. e) Multiple reversible dynamic bonds: the supramolecular polymer network consisting of strong and weak hydrogen bonds and disulfide metathesis. Reproduced with permission.[ 151 ] Copyright 2020, Nature Publishing Group.
