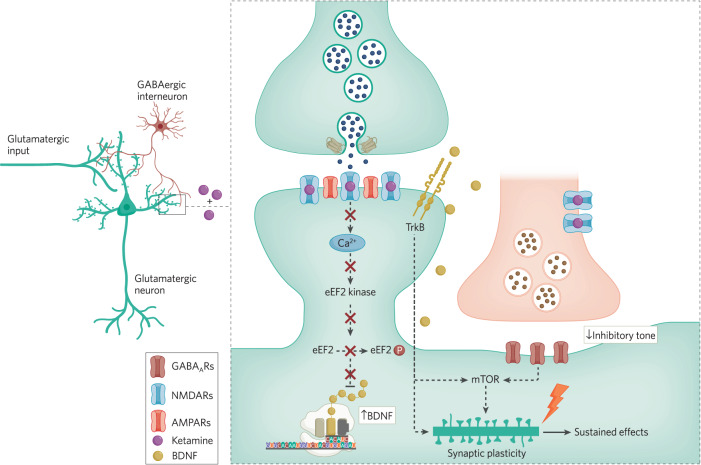
Fig. 1. Converging synaptic signaling pathways underlying ketamine action.
The figure depicts a basic synaptic circuit where an excitatory pyramidal neuron receives inputs from other excitatory neurons as well as inhibitory neurons. At excitatory glutamatergic synapses onto excitatory neurons, glutamate release and NMDA receptor activation leads to activation of eEF2 kinase, triggering eEF2 phosphorylation and silencing of brain-derived neurotrophic factor (BDNF) translation. Ketamine-mediated NMDA receptor blockade, in turn, ceases tonic eEF2 kinase activity, resulting in a gradual loss of eEF2 phosphorylation and de-suppression of BDNF translation, ultimately triggering TrkB receptor signaling. TrkB signaling and subsequent rapid homeostatic synaptic plasticity is required to elicit not only rapid effects of ketamine but also its sustained effects. In the meantime, ketamine-mediated transient reduction of excitatory drive onto inhibitory interneuron resident NMDARs is postulated to suppress tonic release of GABA and disinhibit activity of the target excitatory neurons. The resulting increase in glutamatergic activity produces downstream activation of mammalian target of rapamycin (mTOR) function to elicit structural plasticity and produce the rapid and sustained antidepressant effects. While the two pathways may act synergistically, it is important to note that mTOR may also acts as a downstream target for TrkB signaling, which may be a point of convergence of the two pathways. These initial synaptic plasticity events trigger sustained effects of ketamine via transcriptional processes. This figure was made by BioRender.