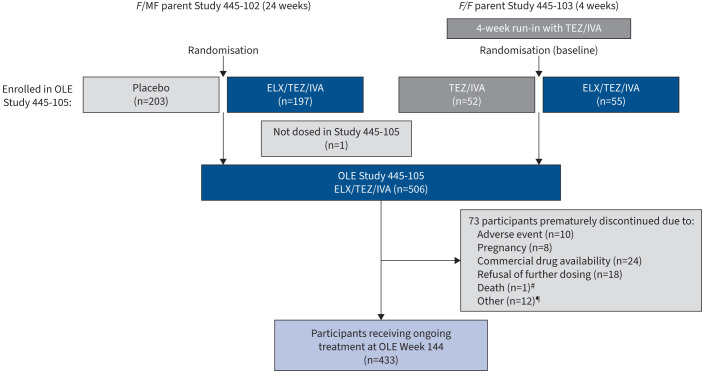
FIGURE 1.
Participant disposition diagram. F: F508del; MF: minimal function; TEZ/IVA: tezacaftor/ivacaftor; OLE: open-label extension; ELX/TEZ/IVA: elexacaftor/tezacaftor/ivacaftor. #: one participant died during the study due to an adverse event of accidental oxycodone toxicity that was considered by the investigator to be unrelated to study drug; ¶: other reasons for discontinuation included physician decision (n=2), requirement of prohibited medication (n=2), loss to follow-up (n=1), noncompliance with study drug (n=1), other noncompliance (n=2) and not specified (n=4).