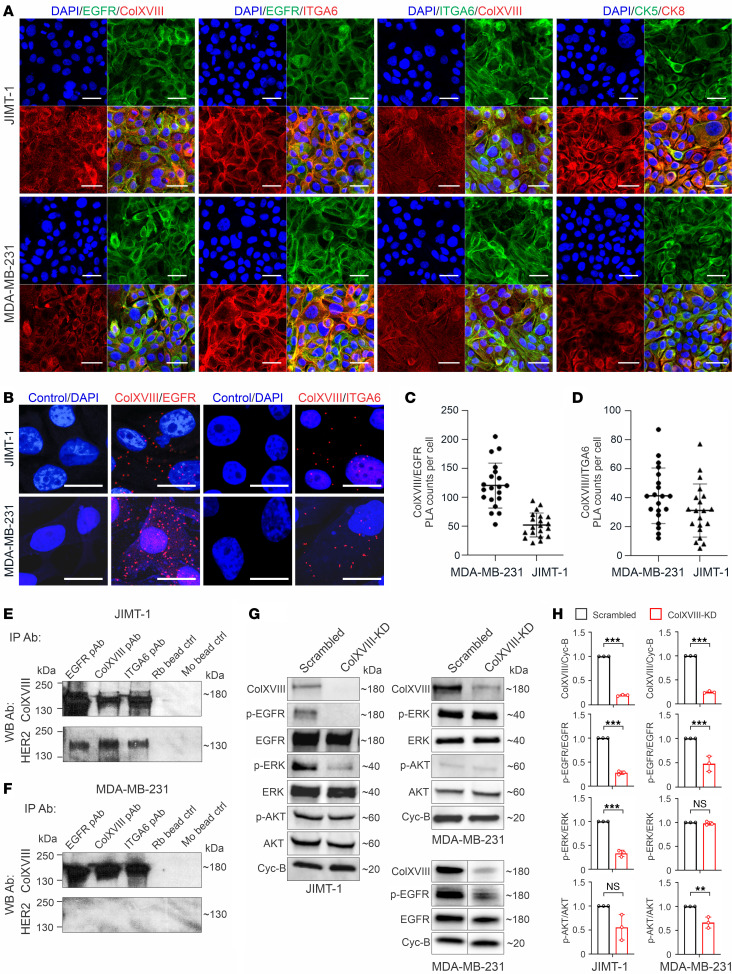
Figure 7. Interactions between ColXVIII, ErbBs, and integrins and analyses of ErbB signaling.
(A) Representative images of immunostaining for ColXVIII, EGFR, and α6-integrin (ITGA6) in JIMT-1 and MDA-MB-231 cells (n ≥3). (B–D) In situ PLA in JIMT-1 and MDA-MB-231 cells. (B) Evidence of proximity (distance < 40 nm) for ColXVIII (mAb DB144-N2) and EGFR (mAb 52894) and for ColXVIII (mAb DB144-N2) and α6-integrin (primary Ab [pAb] 97760) is indicated by the presence of red dots. PLAs without pAbs served as the negative controls. Scale bars: 50 μm (A); 20 μm (B). (C and D) Quantitation of PLA counts for ColXVIII and EGFR (C) and ColXVIII and α6-integrin (D) (n = 3 biological replicates; n = 20 cells per sample). (E and F) Co-IP of ColXVIII (mouse mAB DB144-N2), EGFR (rabbit mAb 52894), and α6-integrin (rabbit pAb 97760) in HER2+ JIMT-1 (E) cells and in triple-negative MDA-MB-231 (F) cells. Protein complexes were detected in Western blot (WB) with ColXVIII (rabbit pAb QH48.18) and HER2 (rabbit mAb 4290) Abs (n ≥ 5). Goat anti–rabbit (Rb) IgG– and goat anti–mouse (Mo) IgG–coated magnetic bead controls are shown. (G) Representative immunoblots of EGFR and downstream signaling mediators in scrambled and ColXVIII-KD JIMT-1 and MDA-MB-231 cell lysates. In MDA-MB-231 cell lysates, the results for p-EGFR and EGFR along with ColXVIII were derived from another biological replicate. (H) Quantitation of ColXVIII, and EGFR, ERK, and AKT phosphorylation in JIMT-1 and MDA-MB-231 cell lysates (n = 3 biological replicates). **P < 0.01 and ***P < 0.001, by 2-tailed Student’s t test (H). Error bars indicate the SD.