Figure 5. Functional model of the role of TRAP in nascent polypeptide processing.
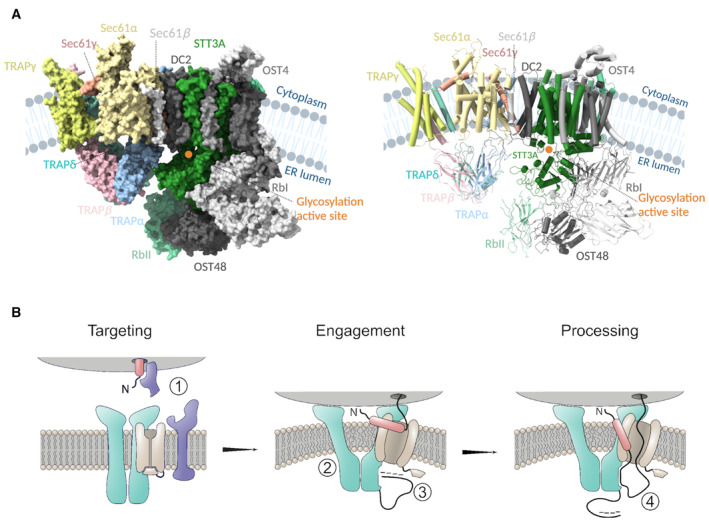
- Cryo‐EM structure of our Sec61/TRAP complex and the structure of OST‐A (PDB ID: 6S7O) complex modeled in the cryo‐ET density of Sec61/TRAP and the OST complex (EMD‐3068), surface (right), and cartoon (left) representation. TRAP subunits colored as TRAPα:cyan, TRAPβ:pink, TRAPδ:green, and TRAPγ: yellow, and Sec61 complex colored as Sec61α:light orange, Sec61β:gray, and Sec61γ:red. Most of the OST subunits are colored in gray except STT3A and RbII which lie in proximity to the lumenal domains of the TRAP complex and are colored green and light green, respectively. The glycosylation active site of the STT3A domain is highlighted with an orange circle. Protein/ribosome structures were rendered with ChimeraX, and the schematics were created with BioRender.com.
- Targeting of the ribosome–nascent chain complex (ribosome in gray, nascent chain's signal peptide in red, and its mature chain in black) is carried out by SRP and SRP receptor (in blue) (1). Docking of the ribosome induces a conformational change in the TRAP/Sec61 complex (TRAP in turquoise and Sec61 in light brown), resulting in membrane perturbation which increases the fluidity of the local lipid environment about the Sec61 lateral gate (2). After successful lateral gate engagement and plug displacement, nascent polypeptide is exposed to the ER lumen, where transient interactions with the negatively charged TRAPα flexible N‐terminal loop (in black) may encourage correct topology and complete lateral gate intercalation of the signal peptide (3). The TRAPα lumenal domain is proximal to the lumenal exit of Sec61, and it may directly bind the nascent polypeptide, serving to prevent back diffusion of translocation inefficient polypeptide sequences (4). Finally, interaction with TRAPα lumenal domain may also serve to transiently restrict the nascent polypeptide for presentation to downstream processing events.
