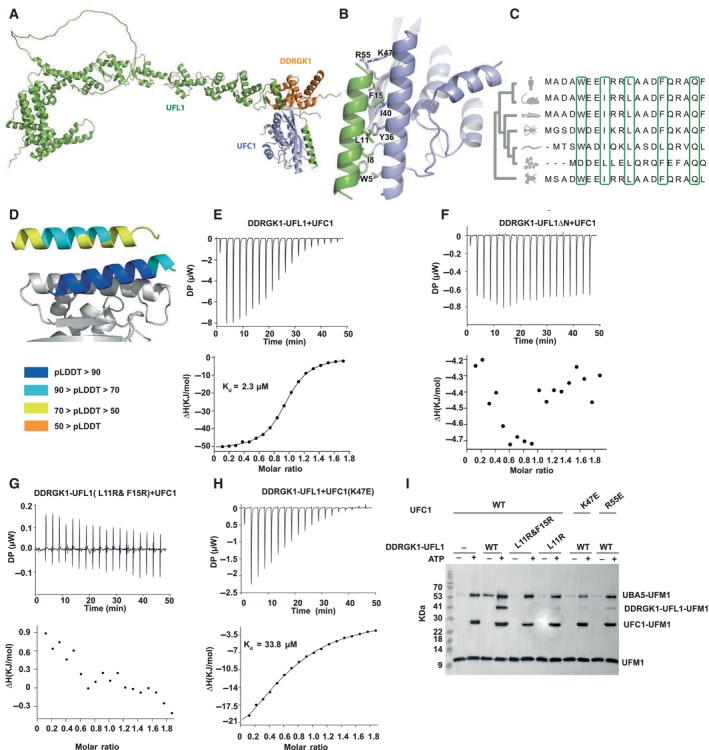
Figure 2. The N‐terminal helix of UFL1 is crucial for binding of UFC1 and activity.
-
AOverall view of the UFC1‐DDRGK1‐UFL1 ternary complex (UFC1 colored in violet).
-
BDetails of the interaction: UFL1 N‐terminal helix bound to UFC1.
-
CAnalysis of conservation of UFL1 N‐terminal helix shows evolutionary conservation of the residues predicted to be involved in binding.
-
DThe N‐terminal helix (colored according to pLDDT) is modeled with high confidence when bound to UFC1 (in gray), in contrast to the low confidence for this helix in the unbound structure (shown on top).
-
E–HITC experiments of UFC1 binding to fusion proteins: (E) UFC1 binding to DDRGK1‐UFL1; (F) UFC1 binding to DDRGK1‐UFL1ΔN (G) UFC1 binding to DDRGK1‐UFL1 double mutant (L11R & F15R); (H) mutant UFC1 (K47E) binding to DDRGK1‐UFL1. In all the experiments, top graphs represent raw data of heat flow versus time. The area under the peaks of the upper panel was integrated and plotted as kJ per mole of UFC1 as a function of binding stoichiometry in the bottom panel. Thermodynamic parameters are summarized in Table EV3.
-
IWestern blot showing the effect of UFC1 mutants or DDRGK1‐UFL1 mutants on ufmylation; blot with anti‐FLAG since UFM1 has FLAG‐tag. See Fig EV2D for protein loading control.
Source data are available online for this figure.
