Figure 5. DDRGK1‐UFL1 exhibits higher affinity for charged UFC1 and enhances destabilization of the latter.
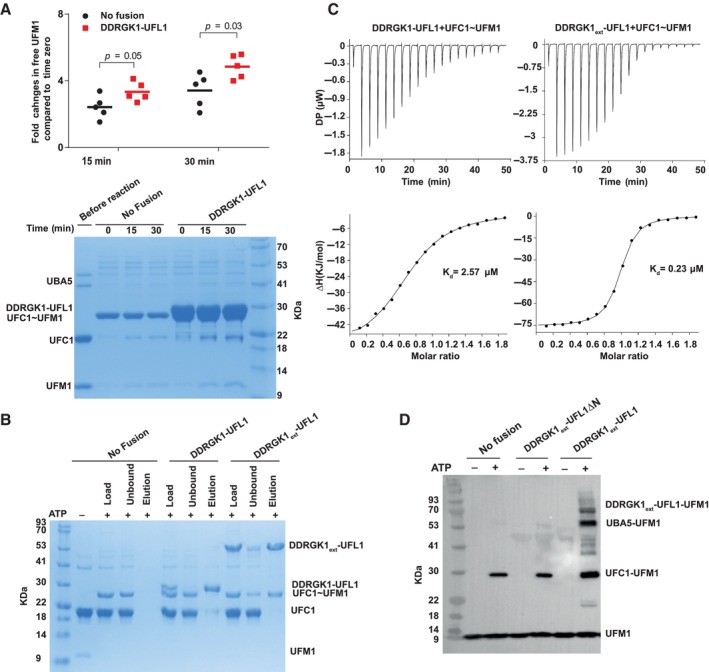
- Single turnover lysine discharge assay showing the effect of DDRGK1‐UFL1 on discharge of UFC1 by free lysine. Five independent experiments were performed to quantify the effect of DDRGK1‐UFL1 on UFC1 discharge. The amount of free UFM1 was quantified at each time point and was normalized against time zero (for 15 min time point pval = 0.05, for 30 min time point pval = 0.03, two‐tailed unpaired Student's t‐test, data as mean ± SD, n = 5 technical replicates). Lower panel: A representative gel showing UCF1~UFM1 discharge upon addition of lysine in the presence and absence of DDRGK1‐UFL1 fusion protein.
- Coomassie stain gel shows that in a pulldown assay DDRGK1ext‐UFL1, but not DDRGK1‐UFL1, preferentially binds to charged UFC1 compared to uncharged UFC1. Specifically, only charged UFC1 is detected in the elution of the pulldown with DDRGK1ext‐UFL1 (last lane).
- ITC experiments of charged UFC1 binding to DDRGK1‐UFL1 (left panel) and DDRGK1ext‐UFL1 (right panel). The top graph represents raw data of heat flow versus time. The area under the peaks of the upper panel was integrated and plotted as kJ per mole of UFC1 as a function of binding stoichiometry in the bottom panel. Thermodynamic parameters are summarized in Table EV3.
- In vitro ufmylation assay. Western blot shows that changes in the ufmylation pattern are only observed in the presence of DDRGK1ext‐UFL1 possessing the UFL1 N‐terminus (blotting with anti‐FLAG). See Fig EV4B for protein loading controls.
Source data are available online for this figure.
