FIG. 2.
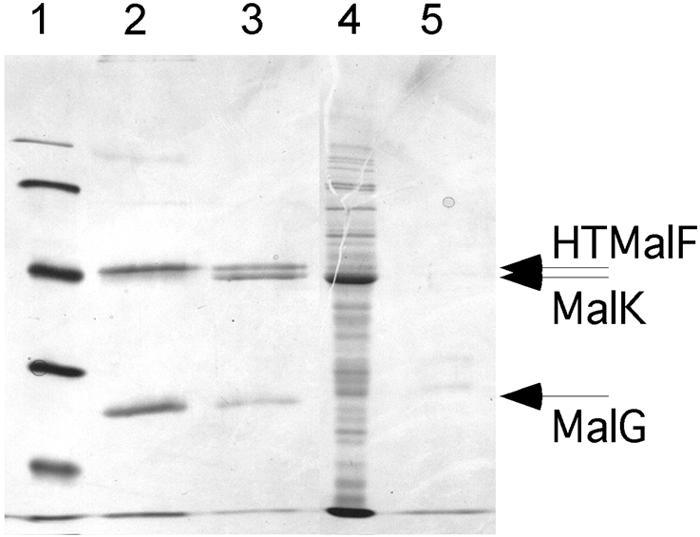
Purification of a MalF-MalG complex and reassembly of MalFGK2. Membranes expressing HTMalF, MalG, and HTMalK(H192R) were treated with 6 M urea to remove HTMalK and then treated with detergent to solubilize HTMalF and MalG. The detergent-soluble fraction was passed over cobalt resin, and purified proteins were eluted with buffer containing100 mM imidazole. Lane 1, molecular weight markers (103) (from top to bottom, 97, 66, 45, 31, and 21.5); lane 2, elution profile showing copurification of HTMalF and MalG; lane 3, elution profile after MalK preparation was passed through the Co resin-containing bound HTMalF/MalG and washed; lane 4, 100,000 × g supernatant fraction containing MalK; lane 5, elution profile after MalK preparation was passed through a Co resin without bound HTMalF/MalG present. Bands are visualized by Coomassie staining.
