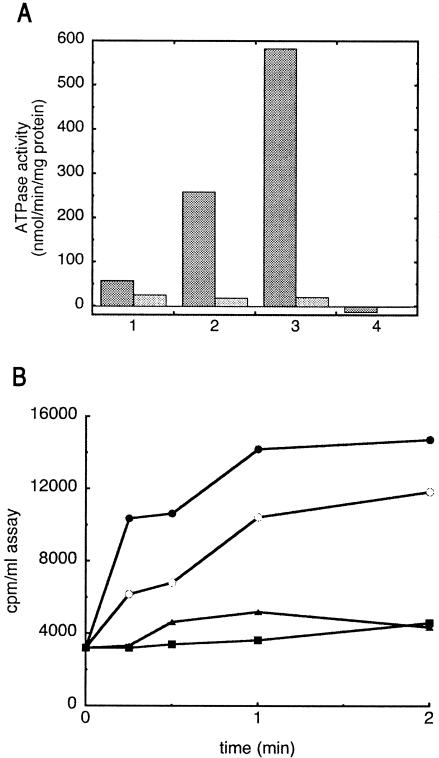
FIG. 3.
Maltose transport and ATPase activity of reassembled transport complexes. A. ATPase activity is measured in the presence (dark bars) or absence (light bars) of 5 μM MBP using 1 mM ATP and 0.1 μM transporter reconstituted in proteoliposomes (3, 5). 1, unpurified MalFG(HTMalK)2; 2, MalFG(HTMalK)2 purified on Co resin; 3, in vitro-reassembled HTMalFGK2 (preparation shown in Fig. 2); 4, purified HTMalF-MalG complex with no MalK (preparation shown in Fig. 2). B. Maltose transport into proteoliposomes is measured with 10 μM maltose, 1 μM MBP, and approximately 0.5 μM transporter (3). Closed circles, in vitro-assembled transporter (initial rate of uptake is 0.9 nmol/min/mg of protein); open circles, in vivo-assembled (unpurified) transporter (0.3 nmol/min/mg of protein); triangles, HTMalF-MalG only; squares, 1 μM MBP only (background).