Abstract
Biobanks often contain several phenotypes relevant to diseases such as major depressive disorder (MDD), with partly distinct genetic architectures. Researchers face complex tradeoffs between shallow (large sample size, low specificity/sensitivity) and deep (small sample size, high specificity/sensitivity) phenotypes, and the optimal choices are often unclear. Here we propose to integrate these phenotypes to combine the benefits of each. We use phenotype imputation to integrate information across hundreds of MDD-relevant phenotypes, which significantly increases genome-wide association study (GWAS) power and polygenic risk score (PRS) prediction accuracy of the deepest available MDD phenotype in UK Biobank, LifetimeMDD. We demonstrate that imputation preserves specificity in its genetic architecture using a novel PRS-based pleiotropy metric. We further find that integration via summary statistics also enhances GWAS power and PRS predictions, but can introduce nonspecific genetic effects depending on input. Our work provides a simple and scalable approach to improve genetic studies in large biobanks by integrating shallow and deep phenotypes.
Subject terms: Genome-wide association studies, Depression
Phenotype imputation increases the effective sample size of major depressive disorder cases in UK Biobank, enhancing study power and polygenic risk score (PRS) accuracy. A new pleiotropy metric enables assessment of PRS specificity and comparison among different PRS models.
Main
Although major depressive disorder (MDD) is the most common psychiatric disorder and the leading cause of disability worldwide, its causes are largely unknown1,2. Despite the moderate familial heritability of MDD (~40%)3, genome-wide association studies (GWASs) have only recently begun to identify replicable risk loci and polygenic risk scores (PRSs)4–9. These discoveries were enabled by increasing power along two primary dimensions: depth of phenotyping and sample size1. Increasing sample size improves both GWAS and PRS power by reducing the standard errors of estimated genetic effects on a given MDD phenotype4,10. Alternatively, increasing diagnostic accuracy through structured clinical interviews prevents dilution of genetic effect sizes, thus improving GWAS power1,9,11 and PRS accuracy11,12. In practice, studies have a fixed budget and must trade off between increasing sample size or phenotyping depth. The optimal choice for current and future MDD studies remains contested11,13,14. Ultimately, the choice will depend on the study’s goals.
One important goal is statistical explanation, defined as the number of GWAS hits or the PRS prediction accuracy. Most MDD GWASs have focused on this goal, which is best achieved by maximizing sample size11,12. This motivates the use of shallow phenotypes in large biobanks, including self-reported depression and treatment5,7. Sample sizes are often further increased by including health record information of seeking care for depression (for example, the Integrative Psychiatric research consortium (iPSYCH15) and the Million Veterans Program8). These studies have amassed sample sizes of millions of individuals and have identified hundreds of risk loci, as well as PRSs with state-of-the-art prediction accuracy in European-ancestry clinical cohorts4–8.
A partly distinct goal is biological insight. This is more difficult to measure or even define, but it represents one of the ultimate goals of genetics: characterizing biological mechanisms to improve prediction and treatment for all. This goal may never be achieved by increasing sample size with shallow phenotyping, because shallow phenotypes are confounded by genetic effects that do not pertain to MDD biology11. In contrast, deep phenotyping in clinical cohorts (for example, the Psychiatric Genomics Consortium (PGC)4 and the China, Oxford and VCU Experimental Research on Genetic Epidemiology (CONVERGE)9) has identified a handful of replicated genetic loci that could potentially generate hypotheses on MDD-specific biology. However, this has not yet been demonstrated robustly, as current sample sizes simply do not provide the power to yield enough genetic signals for definitive biological inferences9.
In this paper, we propose to bridge the shallow–deep gap by integrating information across hundreds of MDD-relevant phenotypes in UK Biobank11,16 (Fig. 1). We focus on using phenotype imputation17,18 to increase the effective sample size for the deepest MDD phenotype in UK Biobank (LifetimeMDD)11, which dramatically improves GWAS power and PRS accuracy over any individual MDD phenotype19. We extensively characterize the genetic architecture underlying these imputed phenotypes and show that they remain specific to LifetimeMDD. Further, we develop a novel approach to partly remove nonspecific signals from GWASs on shallow phenotypes akin to latent factor corrections in expression quantitative trait locus (eQTL) studies20–23. We also investigate phenotype integration via GWAS summary statistics using multi-trait analysis of GWAS (MTAG), which offers varying specificity and sensitivity depending on input choices. Finally, we developed a novel metric to quantify the specificity of a given PRS, which demonstrates that imputed deep phenotypes of MDD are both more specific and more sensitive than observed shallow phenotypes.
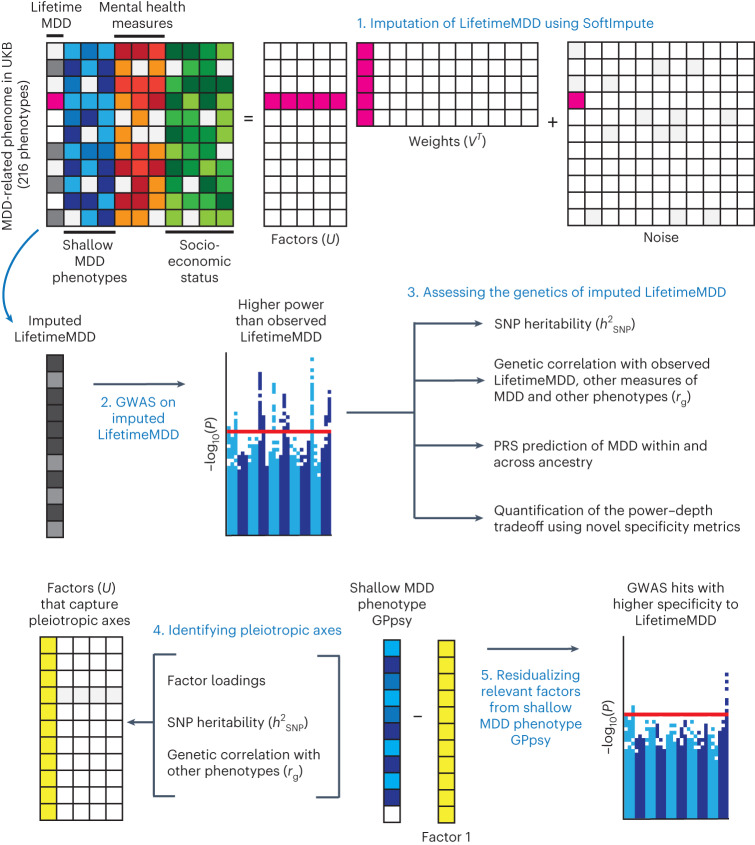
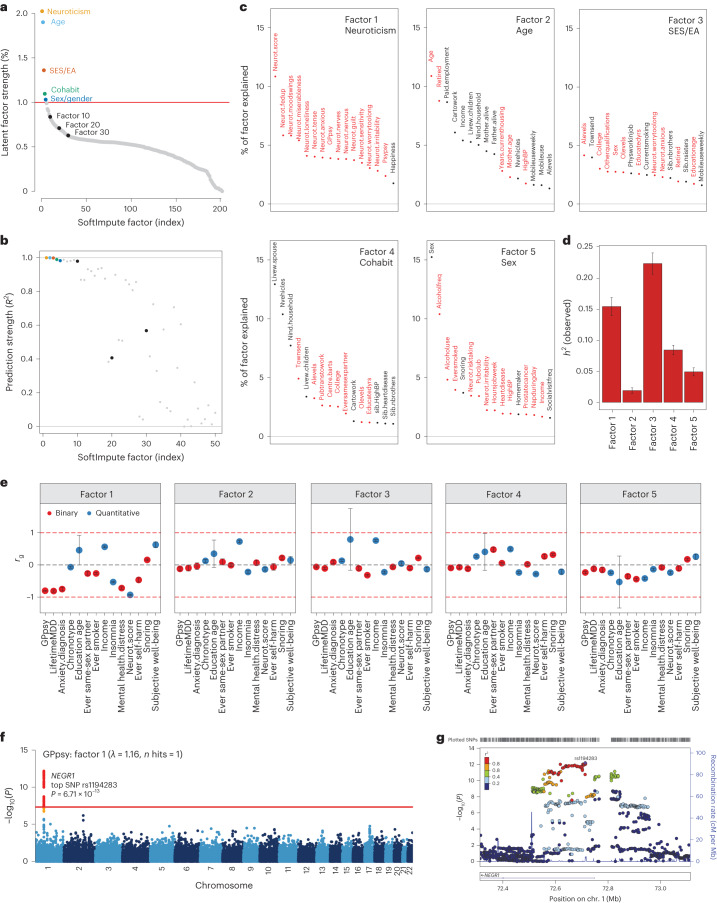
Fig. 1. Study overview.
(1), We imputed LifetimeMDD using a partially observed matrix of depression-relevant phenotypes in UK Biobank (UKB). We focused on using SoftImpute, which also produces latent phenome-wide factors. (2),(3), We then performed GWASs on observed and imputed values of LifetimeMDD (2), as well as downstream polygenic analyses, including in-sample and out-of-sample PRS predictions of MDD (3). (4),(5), We also studied the genetic basis of the latent factors of the depression phenome (4) and residualized latent factors from shallow MDD phenotypes to remove nonspecific pleiotropic effects (5).
Results
Phenotype imputation increases effective sample size
We focused on the deepest available measure of MDD in UK Biobank11, LifetimeMDD, which we derived by applying clinical diagnostic criteria in silico to MDD symptom data from the Patient Health Questionnaire 9 (PHQ9) and the Composite International Diagnostic Interview Short Form (CIDI-SF) in the online Mental Health Questionnaire (MHQ). This procedure identified 16,297 LifetimeMDD cases and 50,867 controls. Because most individuals did not complete these questionnaires, LifetimeMDD was missing for 269,962 individuals. We also studied a shallow measure of MDD, GPpsy11, defined as seeking help from a general practitioner (GP) for “depression, anxiety, tension, or nerves”. For imputation and downstream analyses, we used a broad depression-relevant phenome with 217 phenotypes, including comorbidities and family history, as well as socioeconomic, demographic and environmental phenotypes (Supplementary Note and Supplementary Table 1).
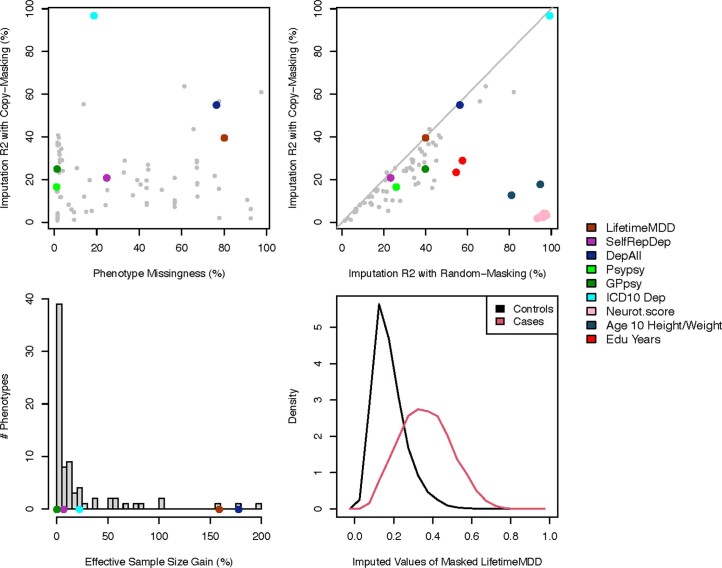
We first imputed the depression phenome using SoftImpute24 (Methods), a variant of principal component analysis (PCA) that identifies latent factors from observed data and uses them to impute missing data. We previously found SoftImpute to be the most scalable among several established approaches17,24. We tuned SoftImpute’s regularization parameter using realistically held-out test data by taking unions of missingness patterns across samples17 and also used this approach to estimate the imputation accuracy for each phenotype (Extended Data Fig. 1)17. Imputation accuracy varied widely across phenotypes, ranging from R2 = 1% for being a twin to R2 = 97% for neuroticism score. For LifetimeMDD (80% missing), we estimated the phenotype imputation R2 to be 40%. This roughly translates to doubling its effective sample size17,25 (nobserved = 67,000, neffective = 166,000; Methods and Extended Data Fig. 1). Imputation accuracy was comparable when stratifying by sex, which is a significant MDD risk factor26–28 (Supplementary Fig. 1 and Supplementary Table 2). We found that the imputed measures had deflated variances and inflated correlations (Supplementary Note and Supplementary Fig. 2), as expected17. This effect could bias some downstream tests, such as tests for genetic correlation. One main goal of this work was to determine whether this approach to phenotype imputation is biased for large-scale single-trait genetic studies.
Extended Data Fig. 1. Imputation accuracy metrics across our depression-relevant UKB phenome.
a, Scatter plot of estimated imputation accuracy against phenotype missingness. b, Scatter plot of estimated imputation accuracy using our copy-masking approach against naive estimates masking entries uniformly at random. c, Distribution across phenotypes of gained effective sample size from phenotype imputation. d, Distribution of imputed LifetimeMDD values for held-out observations, which informally reflect the probabilities of having LifetimeMDD.
Finally, we applied a new deep-learning imputation method, AutoComplete29, to the same phenotype matrix (Methods). AutoComplete improved estimated imputation accuracy for most phenotypes with >10% missingness (29 of 42) and increased the average estimated by R2 by 2.9%.
Phenotype imputation improves GWAS power
We performed GWASs on observed LifetimeMDD (n = 67,164), imputed values of LifetimeMDD (ImpOnly, n = 269,962) and the concatenation of imputed and observed LifetimeMDD (ImpAll, n = 337,126; Fig. 2 and Methods). GWAS on the observed values of LifetimeMDD identified one significant locus (Fig. 2e). In GWASs on the imputed values, the number of GWAS loci increased to 13 and 18 for SoftImpute and AutoComplete, respectively (Fig. 2a, b and Supplementary Table 3). Finally, in GWASs on the combination of both imputed and observed values, the number of significant loci further increased to 26 and 40 for SoftImpute and AutoComplete, respectively (Fig. 2c, d and Supplementary Table 3). We confirmed that these improvements in the number of GWAS hits over the single hit from observed LifetimeMDD were unlikely to occur by chance (Supplementary Fig. 3).
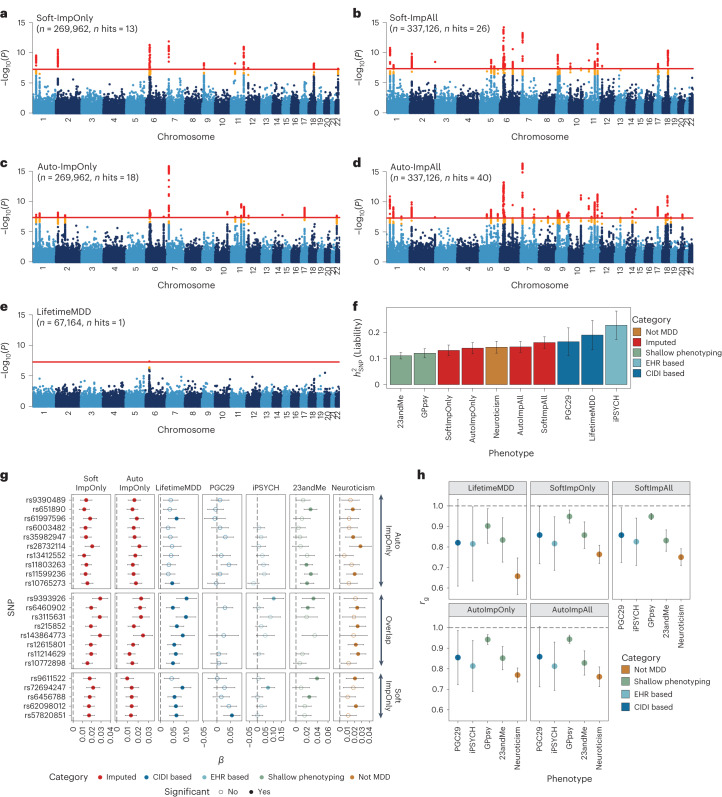
Fig. 2. Genetic architecture of observed and imputed LifetimeMDD.
a–e, Manhattan plots for linear regression GWASs on imputed LifetimeMDD values from SoftImpute (a) and AutoComplete (c) (Soft-ImpOnly and Auto-ImpOnly, n = 269,962) and combined imputed and observed LifetimeMDD values from SoftImpute (b) and AutoComplete (d) (Soft-ImpAll and Auto-ImpAll, n = 337,126) and logistic regression GWAS on observed LifetimeMDD (e) (n = 67,164). −log10(P) values shown on the y axis were before adjustment for multiple testing; red lines show the genome-wide significance threshold of P < 5 × 10−8; P values and test statistics for all GWAS significant SNPs in a–e are shown in Supplementary Table 3. f, Liability-scale estimates of SNP-based heritability and h, genetic correlation between all UK Biobank phenotypes (n values for Soft, Auto and LifetimeMDD GWASs as above; GPpsy n = 332,629; neuroticism n = 274,056) and external MDD studies from PGC (n = 42,455), iPSYCH (n = 38,128) and 23andMe (n = 307,354). g, Replication of GWAS effect sizes from Soft-ImpOnly and Auto-ImpOnly in observed LifetimeMDD and external MDD studies. All error bars indicate 95% CI.
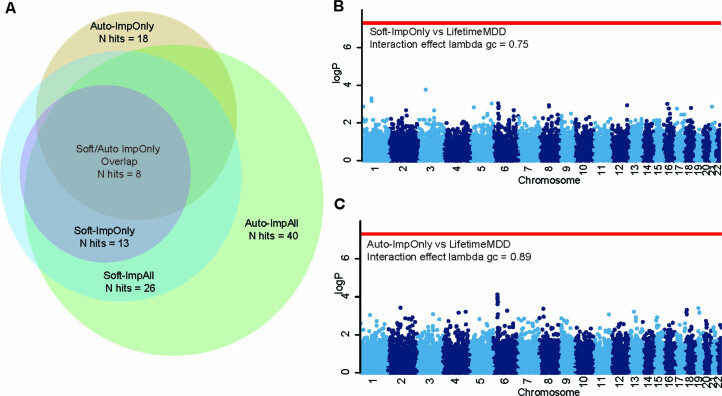
We investigated whether the new GWAS hits from phenotype imputation were MDD specific by comparing the ImpOnly GWASs to other MDD GWASs. First, we compared the two imputation methods. Of the 13 and 18 ImpOnly GWAS loci for SoftImpute and AutoComplete, respectively, 8 overlapped (giving a total of 23; Extended Data Fig. 2). Further, 9 of the remaining 15 loci had P < 10−5 in both ImpOnly GWASs, and all 15 of the 23 loci had P < 0.05/23. Overall, our two imputation methods captured highly overlapping genetic signals, but AutoComplete identified more loci. Next, we assessed the eight shared hits in four nonoverlapping depression cohorts (Methods and Supplementary Note): observed LifetimeMDD in UK Biobank, self-reported depression diagnosis or treatment in 23andMe7, the 29 MDD cohorts of the PGC4 (PGC29) and Danish registry data on MDD cases and population controls (iPSYCH15,30). For reference, we also compared our findings to those for neuroticism in UK Biobank, a personality trait that is genetically correlated with, but distinct from, MDD31. We found that all eight hits shared by both ImpOnly GWASs had sign-consistent effect sizes across all four depression cohorts, as well as in neuroticism. Moreover, all eight hits had P < 0.05/23 for observed LifetimeMDD in UK Biobank. Finally, of the 23 single-nucleotide polymorphisms (SNPs) significant in either of the ImpOnly GWASs, 18 replicated in at least one GWAS of observed MDD at P < 0.05/23 (Fig. 2g). Altogether, these results show that the loci underlying imputed LifetimeMDD are relevant to MDD.
Extended Data Fig. 2. Quality control on GWAS loci identified from imputed LifetimeMDD.
a, Venn diagram showing the overlap of GWAS loci identified from GWAS on ImpOnly and ImpAll measures of LifetimeMDD from Softimpute and Autocomplete. b,c, Manhattan plots of Cochran′s Q statistic’s P-value for heterogeneity, obtained through a random effect meta-analysis performed with METASOFT, between genetic effects identified from GWAS on observed LifetimeMDD and GWAS on ImpOnly measures of LifetimeMDD; −log10(P) values shown on the y-axis were before adjustment of multiple-testing; red line shows the genome-wide significance threshold corresponding to P = 5 × 10−8.
We then checked whether the ImpOnly GWAS preserved the polygenic architecture of LifetimeMDD in terms of heritability and genetic correlation. First, we found that the liability-scale SNP-based heritability ( from LDSC32) was lower for imputed LifetimeMDD (Soft-ImpOnly = 13.1%, standard error (SE) = 1.0%; Auto-ImpOnly = 14.0%, SE = 1.1%) than for observed LifetimeMDD ( = 19.0%, SE = 2.9%; Fig. 2f). Nonetheless, the genetic correlations between imputed and observed LifetimeMDD were near 1 (Soft-ImpOnly: rg = 0.97, SE = 0.02; Auto-ImpOnly: rg = 0.96, SE = 0.03), as it was between the two imputation methods (rg = 1.00, SE = 0.004). Moreover, the rg between the ImpOnly phenotypes and other depression phenotypes largely mirrored the rg based on observed LifetimeMDD (Fig. 2h).
Finally, we tested for effect size heterogeneity between the ImpOnly and observed LifetimeMDD GWASs. We used a random-effect meta-analysis33 (Methods), as the ImpOnly and observed LifetimeMDD GWASs used nonoverlapping individuals. We found no significant heterogeneity in effect size between ImpOnly and observed LifetimeMDD at genome-wide significance (Extended Data Fig. 2), and across the 13 and 18 GWAS hits in Soft-ImpOnly and Auto-ImpOnly, respectively, 6 and 4 SNPs showed significant heterogeneity at P < 0.05/23. Altogether, our results show that imputed LifetimeMDD is noisier than observed LifetimeMDD but captures similar genetic effects.
Phenome-wide factors index pleiotropic axes of depression risk
We examined the top latent factors in SoftImpute to understand what phenotypic correlations drive the imputation. We used two statistical metrics to prioritize significant factors. First, we quantified the phenome-wide variance explained (Methods and Fig. 3a): the top handful of factors clearly stood out, with factors becoming comparable to background noise levels around factor 30. Second, we quantified factor stability by calculating the R2 between factors estimated on separate halves of the data, similar to prediction strength in clustering34 (Methods and Fig. 3b). We found that the first ten factors were very stable (min R2 of ~ 98%), with stability decaying steadily thereafter (factors 11–20, average R2 of ~ 80%; factors 21–30, average R2 of ~60%). We conservatively conclude that the first ten or so factors are statistically meaningful.
Fig. 3. Characterizing top latent factors driving SoftImpute.
a,b, Statistical importance of each factor measured by percentage variance explained in the phenotype matrix (a) and factor prediction strength (b). c, Top phenotype loadings for the top five SoftImpute factors. d,e, Estimates of heritability (d) (n = 337,126 for all factors) and genetic correlation (e) of the top five SoftImpute factors with MDD-relevant traits (n values for all MDD-relevant traits can be found in Supplementary Table 1). f, Logistic regression GWAS Manhattan plot of GPpsy conditioning on SoftImpute factor 1; the red line shows the genome-wide significance threshold. g, LocusZoom plot of the significant GWAS locus in gene NEGR1. All error bars indicate 95% CI.
We studied the genetic basis of each factor with GWASs. The number of GWAS hits ranged from 3 (factor 2, mainly indexing age-related phenotypes; Fig. 3c) to 309 (factor 3, mainly indexing socioeconomic status and education attainment), with λGC ranging from 1.15 (factor 2) to 2.11 (factor 3) (Supplementary Fig. 5). We next estimated heritabilities and found they ranged from = 1.9% (SE = 0.2%) for factor 2 to = 22.4% (SE = 0.9%) for factor 3 (Fig. 3d and Supplementary Fig. 4). Finally, we profiled the genetic correlation between factors and MDD-related phenotypes (Fig. 3e and Supplementary Fig. 4). We found that the genetic correlations closely mirrored the factor loadings, which are based on phenotypic correlations. For example, factor 1 had rg = −0.93 (SE = 0.01) with neuroticism and factor 3 was correlated with years of education (rg = 0.79 (SE = 0.96) and income (rg = 0.75, SE = 0.03).
Given these results, we hypothesized that our top phenome-wide factors partly capture nonspecific pathways contributing to shallow MDD phenotypes. To test this hypothesis, we performed GWAS analysis on a shallow MDD measure, GPpsy (n = 332,629), conditional on factor 1. This is akin to removing confounders such as batch effects in eQTL studies through conditioning on latent factors. We found that only 1 of the 25 GWAS hits for GPpsy remained after adjusting for factor 1 (Fig. 3f). This hit overlaps NEGR1 (top SNP rs1194283, odds ratio (OR) = 1.05, SE = 0.0065, P = 6.71 × 10−13; Fig. 3g), which has been identified as an MDD risk locus in multiple GWASs with varying phenotyping approaches4,6,8,35–37. Intriguingly, this locus also has replicated associations with body mass index and obesity in multiple populations38–41. We also performed GWASs conditioned on each of the other top ten factors, which generally had little impact (Supplementary Fig. 6). One clear exception, however, was that adjusting for factor 3 increased the number of GPpsy GWAS hits from 25 to 35. These additional loci could be false positives from collider bias23,42,43.
MTAG improves GWAS power but is sensitive to inputs
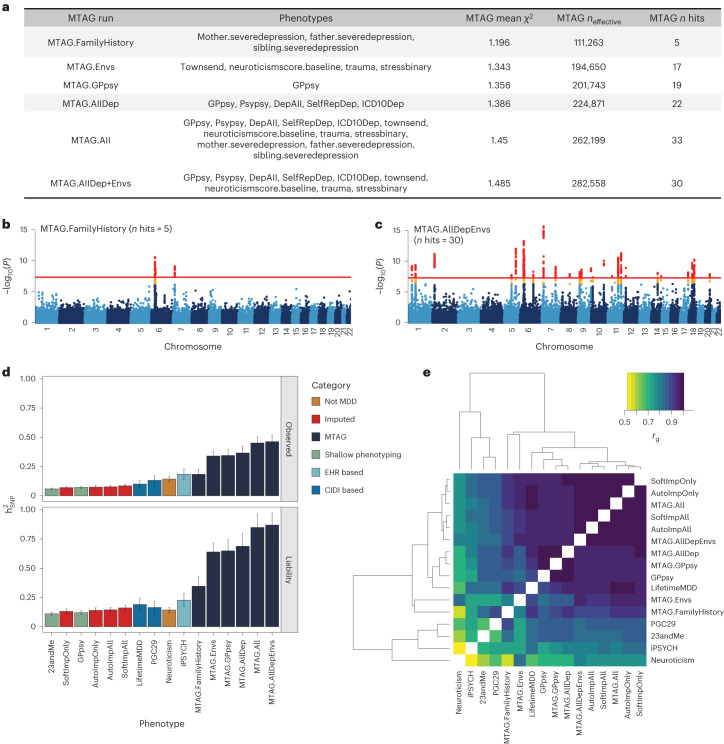
As an alternative to phenotype imputation, we next evaluated phenotype integration at the GWAS summary statistic level using MTAG19, an inverse-covariance-weighted meta-analysis for GWAS on multiple traits. We did not use all 217 phenotypes in MTAG for two reasons. First, MTAG requires GWAS to be run on each input phenotype, which is computationally intractable for hundreds of phenotypes. Second, MTAG produces false positives when applied to large numbers of input GWAS19. Instead, we evaluated six different sets of input GWAS to MTAG, producing six different integrated LifetimeMDD GWASs (Fig. 4a, Extended Data Fig. 3, Supplementary Table 4 and Supplementary Note).
Fig. 4. MTAG results for different choices of input phenotypes.
a, Description of the evaluated input choices for MTAG and their resulting GWAS summary statistics. The MTAG effective sample size refers to the power-equivalent sample size of the MTAG GWAS versus the single-trait GWAS19, calculated as neffect = nsingle ⨯ (χ2MTAG − 1)/(χ2single − 1), where the χ2 terms are the average GWAS chi-squared values. b,c, Manhattan plots for the MTAG models with the fewest (MTAG.FamilyHistory) (b) and most (MTAG.AllDep+Envs) (c) GWAS hits. −log10(P) values shown on the y axis were before adjustment for multiple testing; red lines show the genome-wide significance threshold of P < 5 × 10−8. d, SNP-based heritability estimates on the observed and liability scales for observed, imputed, and MTAG GWASs on LifetimeMDD as well as reference phenotypes (neffect is shown in Supplementary Table 5). e, Estimated genetic correlations for observed, imputed and MTAG analyses of LifetimeMDD and reference phenotypes. All error bars indicate 95% CI.
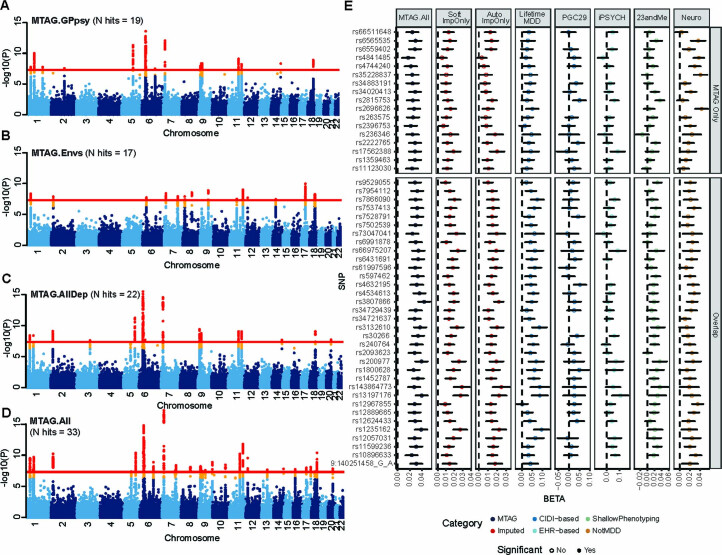
Extended Data Fig. 3. MTAG GWAS and genetic architecture.
a-d, Manhattan plots showing MTAG results for LifetimeMDD for the MTAG runs, MTAG.GPpsy, MTAG.Envs, MTAG.AllDep and MTAG.All, which are described in Fig. 4a; red line shows the genome-wide significance threshold (P = 5 × 10−8). e, Replication of LifetimeMDD GWAS effect sizes for loci identified only by MTAG and those identified by bothMTAG and imputation (Softimpute or Autocomplete). Effect sizes are shown for observed LifetimeMDD and external MDD studies from PGC (n = 42,455), iPSYCH (n = 38,128), and 23andMe (n = 307,354). All error bars indicate 95% confidence intervals.
All six MTAG input choices increased the number of GWAS hits from observed LifetimeMDD. On the low end, MTAG using family history measures of depression yielded five GWAS hits (MTAG.FamilyHistory, λGC = 1.20; Fig. 4b). On the high end, MTAG using shallow MDD phenotypes and environmental factors (such as recent stressful life events, lifetime traumatic experiences and the Townsend deprivation index) yielded 33 GWAS hits (MTAG.All, λGC = 1.45; Fig. 4c). We note that MTAG.AllDep is analogous to prior depression phenotypes defined by manually combining similar depression measures14 and that MTAG.FamilyHistory is analogous to prior methods that integrated family history into GWAS analysis44,45. Of the 51 total hits across all MTAG runs, 34 overlapped hits from the imputed GWASs (Extended Data Fig. 3). Notably, we found that adding more input phenotypes in MTAG always increased the number of GWAS hits. This reflects a combination of added power by leveraging pleiotropy and an increased false positive rate19. Additionally, MTAG GWASs yielded substantially inflated heritability estimates (on both the liability and observed scales), which increased with more input GWAS. For example, MTAG.All gave = 84.9% (SE = 5.6%), compared to = 19.0% (SE = 2.9%) for observed LifetimeMDD (Fig. 4d and Supplementary Table 5).
We next examined genetic correlations between MTAG and other MDD GWASs (Fig. 4e). First, MTAG.All, which included the most input GWAS, clustered together with the imputed GWASs, which leveraged all 217 phenotypes. Second, MTAG using shallow MDD phenotypes (MTAG.AllDep and MTAG.GPpsy) clustered with the GWAS on GPpsy. Third, neuroticism was significantly more genetically correlated with MTAG.Envs (rg = 0.84, SE = 0.01) than LifetimeMDD ( = 0.66, SE = 0.06). These results are consistent with prior observations that MTAG-based summary statistics modestly inflate genetic correlation with the input phenotypes46. Overall, the genetic correlations between MTAG and LifetimeMDD were high, with the lowest value derived for MTAG.Envs (rg = 0.90, SE = 0.03).
Finally, to compare like to like, we evaluated SoftImpute’s accuracy using only the MTAG.All input phenotypes (plus sex, age and 20 PCs). We found that imputation performed much worse with this reduced set of phenotypes (phenome-wide R2 decreased from 59.6% to 39.5%, P < 2 × 10−5, pooled t-test across folds; Supplementary Fig. 2 and Supplementary Table 2).
Phenotype imputation and MTAG improves PRS accuracy
We then assessed the within-sample prediction accuracy of PRSs built from integrated MDD GWAS. We used ten-fold cross-validation to obtain the Nagelkerke’s prediction accuracy for LifetimeMDD in white British individuals in UK Biobank. We jointly cross-validated the phenotype imputation and PRS construction (Methods). For MTAG, we jointly cross-validated the GWASs on secondary input phenotypes. For context, we compared these PRSs to ones built from observed LifetimeMDD (n = 67,164; for neffect see Supplementary Table 6) and GPpsy (n = 332,629) in UK Biobank11, MDD defined by structured interviews in PGC29 (ref. 4) (n = 42,455), affective disorder defined by Danish health registries in iPSYCH15 (n = 38,123) and self-reported depression in 23andMe7 (n = 307,354; Supplementary Table 6).
We found that imputing LifetimeMDD doubled PRS prediction accuracy over observed LifetimeMDD (Fig. 5a; LifetimeMDD: R2 = 1.0%, 95% confidence interval (CI) = 0.6–1.4%; Soft-ImpAll: R2 = 2.1%, 95% CI = 1.3–2.9%; Auto-ImpAll: R2 = 2.2%, 95% CI = 1.4–3.0%). Consistent with prior reports11,12, we found that the GPpsy PRS predicted LifetimeMDD better than the LifetimeMDD PRS itself (R2 = 1.6%, 95% CI = 0.6%–2.4%) because it has a roughly four times larger sample size. Nonetheless, both the SoftImpute and AutoComplete PRSs outperformed the GPpsy PRS, demonstrating that integrating shallow and deep phenotypes through imputation can improve PRS accuracy over that with either class of phenotypes alone. Finally, we found that the imputed LifetimeMDD PRS substantially outperformed the PRSs from iPSYCH (R2 = 0.6%, 95% CI = 0.2–0.9%) and 23andMe (R2 = 1.3%, 95% CI = 0.7–1.9%), even though iPSYCH used deeper phenotypes and 23andMe had a large sample size.
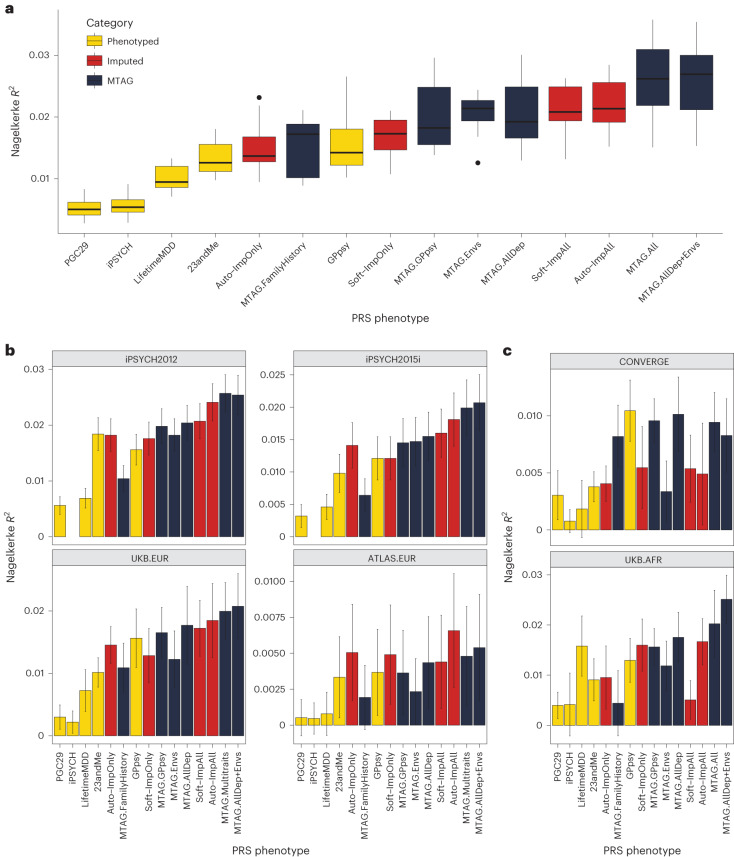
Fig. 5. PRS performance using observed, imputed and/or meta-analyzed MDD.
a, PRS prediction accuracy for LifetimeMDD (n = 67,164) in unrelated white British individuals in UK Biobank using ten-fold cross-validation. For the imputed PRSs, we also cross-validated the imputation. Median values are shown as a line in the box; whiskers of boxplots are 1.5 times the interquartile range; outliers outside of the interquartile range are shown as filled dots. b, Out-of-sample PRS prediction accuracy in four additional cohorts with European ancestries (iPSYCH2012, n = 42,250; iPSYCH2015i, n = 23,351; UKB.EUR, n = 10,193; ATLAS.EUR, n = 14,366). c, PRS prediction accuracy in African-ancestry individuals in UK Biobank (n = 687) and Han Chinese-ancestry individuals in CONVERGE (n = 10,502). All error bars indicate 95% CI.
The performance of the MTAG PRSs mirrored the MTAG GWAS results and depended on the input phenotypes (Fig. 4 and Extended Data Fig. 3). First, MTAG.FamilyHistory showed improved accuracy compared to observed LifetimeMDD but underperformed in comparison to the imputed PRSs (R2 = 1.5%, 95% CI = 0.6–2.5%; Fig. 5a). On the other hand, MTAG.All outperformed the imputed PRSs by about 20% (R2 = 2.6%, 95% CI = [1.3%, 3.9%]; Fig. 5a). In particular, this demonstrates that MTAG with more than ten inputs, which is nonstandard and likely yields miscalibrated GWAS results, can significantly improve PRS prediction.
Phenotype imputation and MTAG improves PRS portability
We next asked whether phenotype integration improves PRS predictions in different cohorts, diagnostic systems and/or populations. Demonstrating portability is essential to establish that phenotype integration does not merely reflect dataset-specific biases.
First, we tested PRS accuracy in non-British European-ancestry individuals in UK Biobank (UKB.EUR, n = 10,166). These individuals were measured on the same LifetimeMDD phenotype as our sample of white British UK Biobank individuals and also have European ancestry, and hence represent the most similar cohort (Supplementary Note and Supplementary Fig. 7). Although the small sample size limits definitive conclusions, we observed a nearly identical pattern among the PRS methods as in our training sample: imputation and MTAG almost always improved prediction accuracy compared to both LifetimeMDD and GPpsy (Fig. 5b). We next assessed portability to two large European-ancestry cohorts from iPSYCH (2012 cohort (n = 42,250) and 2015i cohort (n = 23,351); Supplementary Note). These nonoverlapping samples were drawn from a nationwide Danish birth cohort with diagnoses obtained from national health registers30,47. We again found qualitatively identical results, with imputation outperforming both LifetimeMDD and GPpsy and the best MTAG setting outperforming imputation (Fig. 5b). Finally, we tested portability to European-ancestry individuals in the ATLAS dataset based on MDD as defined in University of California - Los Angeles electronic health record (EHR) data48,49 (ATLAS.EUR, n = 14,388; Supplementary Note, Supplementary Fig. 8 and Supplementary Tables 6–8). Again, the small sample size prevented definitive comparisons, but phenotype imputation and the best MTAG setting improved estimated accuracy (Fig. 5b).
We next tested these PRSs in individuals with non-European genetic ancestries, including African-ancestry individuals7 in UK Biobank with observed LifetimeMDD status (UKB.AFR, n = 687), as well as Han Chinese-ancestry individuals in the CONVERGE cohort9,50 (n = 10,502; Supplementary Note) who were assessed for severe, recurrent MDD (Fig. 5c, Supplementary Note and Supplementary Table 6). Consistent with previous studies51–53, we found that the PRSs we derived from GWASs on European-ancestry cohorts generally had poorer portability to non-European-ancestry cohorts, making firm conclusions difficult. Nonetheless, the best MTAG setting was always nearly optimal, and PRSs based on imputed LifetimeMDD always outperformed the PRS based on observed LifetimeMDD. Finally, we tested PRS prediction accuracy in UK Biobank individuals with Asian ancestry (UKB.ASN, n = 334), as well as ATLAS individuals who self-identified as Latino (ATLAS.LAT, n = 2,454), Black (ATLAS.AFR, n = 1,158) or Asian (ATLAS.ASN, n = 1,996). However, the power was too low in these small cohorts for meaningful interpretation (Supplementary Fig. 9).
A new metric for the specificity of PRSs
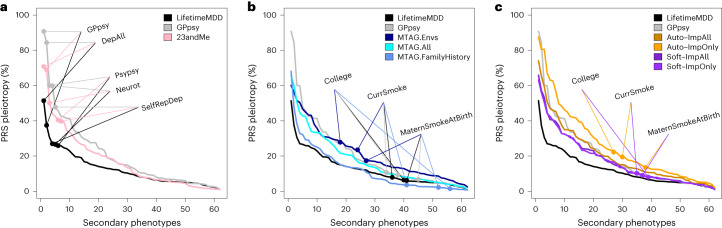
While phenotype integration improves PRS prediction in UK Biobank and in external cohorts, this may come at the cost of reduced specificity to MDD. This is because integration explicitly borrows information from secondary phenotypes, which could introduce genetic signals that are not specific to MDD. To quantify this spillover of nonspecific effects into an MDD PRS, we compared prediction accuracy for LifetimeMDD to prediction accuracy for secondary phenotypes. We call this metric of specificity ‘PRS pleiotropy’ (R2secondary/R2LifetimeMDD). Because core MDD biology is likely partly pleiotropic, its PRS pleiotropy should be nonzero for many secondary phenotypes. We further expect that shallow MDD phenotypes, such as GPpsy, would generally have higher PRS pleiotropy than LifetimeMDD11.
For all PRSs based on observed and integrated MDD, we calculated PRS pleiotropy for 172 secondary phenotypes used in imputation. We then investigated the 62 secondary phenotypes that were significantly predicted by any examined PRS (P < 0.05/172; Methods). Visualizing PRS pleiotropy for observed LifetimeMDD across this depression phenome showed a spectrum of highly linked traits, including shallow MDD phenotypes such as GPpsy and genetically correlated traits such as neuroticism, that quickly faded across successive phenotypes (Fig. 6a). By comparison, GPpsy broadly had higher PRS pleiotropy across secondary phenotypes, indicating that GPpsy captures less-specific biology than LifetimeMDD, as expected. We also found that the 23andMe GWAS had similar PRS pleiotropy to GPpsy, consistent with the fact that both measure MDD by self-reported depression.
Fig. 6. Phenome-wide PRS pleiotropy quantifies nonspecificity.
PRS pleiotropy spectra across the depression-relevant phenome, defined as the ratio of PRS prediction accuracy for secondary traits relative to LifetimeMDD (PRS pleiotropy = R2secondary/R2LifetimeMDD). a, PRSs derived from GWAS on shallow MDD phenotypes (GPpsy or 23andMe) are less specific to LifetimeMDD than the PRS derived from the GWASs on LifetimeMDD. b, MTAG-based PRSs range from highly specific (MTAG.FamilyHistory) to less specific (MTAG.Envs) compared to PRSs derived from shallow MDD phenotypes. c, SoftImpute PRSs are more specific than PRSs derived from shallow MDD phenotypes, while AutoComplete PRSs are similar. Note that GPpsy and LifetimeMDD are each used in two ways: (1) to build the PRS and (2) to evaluate PRS pleiotropy.
Our main question here was whether phenotype integration has more PRS pleiotropy than self-reported depression as in GPpsy and 23andMe. We first evaluated PRS pleiotropy for MTAG and found that specificity highly depended on the input GWAS (Fig. 6b). First, MTAG.Envs had far higher PRS pleiotropy than GPpsy, showing that its high PRS power comes at a high cost to specificity. On the other hand, MTAG.All had a similar PRS pleiotropy as GPpsy and nearly double the PRS R2; hence, MTAG.All is clearly superior to GPpsy. MTAG.FamilyHistory had the opposite properties: it only modestly improved PRS R2 over observed LifetimeMDD, but this benefit came without loss of specificity. We then evaluated PRS pleiotropy for imputed phenotypes (Fig. 6c). The SoftImpute ImpAll and ImpOnly PRSs were both more specific to LifetimeMDD than the GPpsy PRS, which is notable given that the imputed values were constructed from more than 200 phenotypes, including GPpsy. The AutoComplete ImpOnly PRS was less specific than GPpsy, although the ImpAll PRS was comparable.
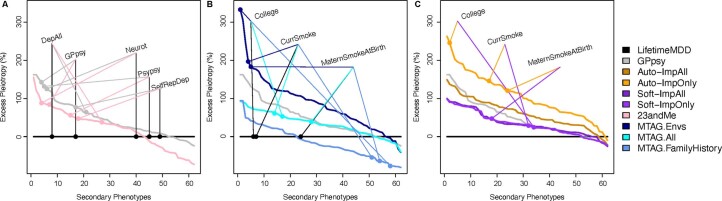
Finally, we evaluated PRS pleiotropy relative to observed LifetimeMDD to ask which of the phenotypes had excess pleiotropy in the integrated PRSs. We defined ‘excess PRS pleiotropy’ as the PRS pleiotropy minus the PRS pleiotropy of observed LifetimeMDD11. As expected, self-reported depression PRSs (GPpsy and 23andMe) had high excess PRS pleiotropy, especially when compared to shallow MDD measures (Extended Data Fig. 4a). Likewise, MTAG.Envs had substantial excess PRS pleiotropy, especially for socioeconomic measures such as years of education (Extended Data Fig. 4b). Notably, MTAG.FamilyHistory had far less excess PRS pleiotropy than other MTAG settings or GPpsy, as well as reduced pleiotropy for several socioeconomic measures. Finally, SoftImp-All had lower excess PRS pleiotropy than GPpsy (41/62 phenotypes); however, AutoImp-All had higher excess PRS pleiotropy (Extended Data Fig. 4c). Overall, MTAG can outperform imputation in PRS sensitivity or specificity depending on input, while imputation provides a simple and scalable approach that performs well in both power and specificity.
Extended Data Fig. 4. Excess PRS Pleiotropy.
Excess PRS Pleiotropy measures the pleiotropy of a given PRS relative to the PRS from observed LifetimeMDD. PRS Pleiotropy is defined as the PRS prediction ratio for a secondary trait relative to observed LifetimeMDD (PRS Pleiotropy = R2secondary/R2LifetimeMDD), and excess pleiotropy is the increase relative to the LifetimeMDD PRS (Excess PRS Pleiotropy = (PRS Pleiotropy − LifetimeMDD PRS Pleiotropy)/LifetimeMDD PRS Pleiotropy). Plots are ordered by Excess PRS Pleiotropy for each PRS. a, The PRS derived from GPpsy and 23andMe are less specific to LifetimeMDD than the observed LifetimeMDD PRS, especially for shallow MDD phenotypes and neuroticism. b, MTAG.Envs has high Excess PRS Pleiotropy to secondary traits like college education, smoking, and maternal smoking, while MTAG.FamilyHistory actually reduces PRS Pleiotropy for these traits. c, Both ImpOnly and ImpAll SoftImpute phenotypes show lower Excess PRS Pleiotropy than GPpsy, and the ImpAll GWAS from Autocomplete is comparable to GPpsy.
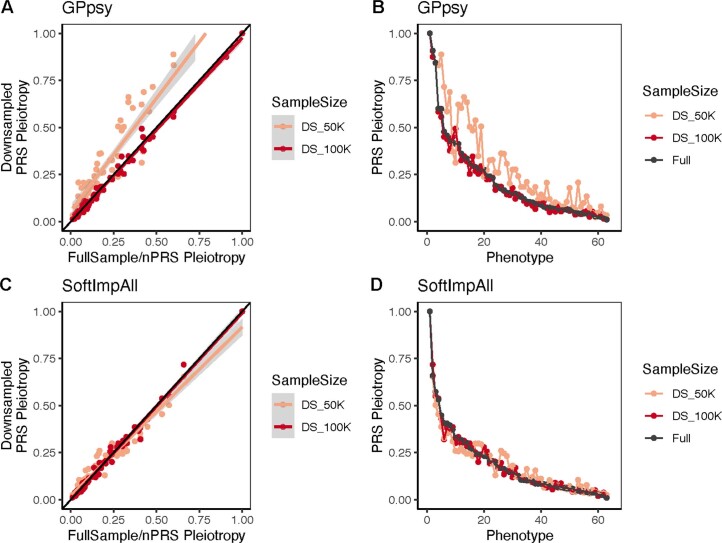
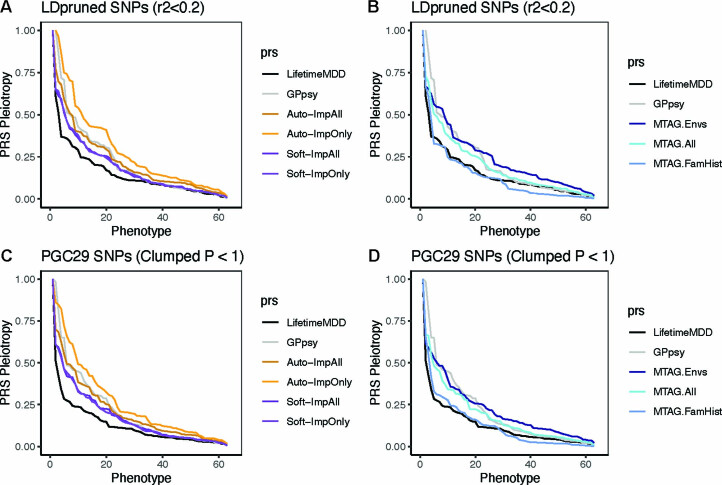
We then downsampled each GWAS used to build a PRS to assess the impact of sample size (Supplementary Note). Overall, we found that PRS pleiotropy was stable, although it can be upwardly biased for sample sizes below 100,000 (due to low power). In particular, our results were robust to differences in the training PRS sample sizes, except that observed LifetimeMDD PRS pleiotropy is a conservative baseline because it is trained on 67,000 individuals (Extended Data Fig. 5 and Supplementary Figs. 10 and 11). Further, we confirmed that these results persisted when we used the same SNPs in each PRS (Supplementary Note and Extended Data Fig. 6).
Extended Data Fig. 5. PRS Pleiotropy is robust to GWAS sample size in practice.
a-d, PRS Pleiotropy of GPpsy (n = 332,629) (a,b) and Soft-ImpAll (n = 337,126) (c,d) at full sample size and down-sampled to n = 50K and 100K for 62 phenotypes in UKB. Plotted values show the mean PRS Pleiotropy from 10-fold cross-validation in UKB.
Extended Data Fig. 6. PRS Pleiotropy is robust to SNP pruning.
a-d, PRS Pleiotropy of imputed and MTAG GWAS for 62 phenotypes in UKB (which are significantly predicted by at least one full-sample PRS, as shown in Fig. 6), where the PRS is constructed with 136,563 LD-pruned SNPs (r2 < 0.2) in UKB (a,b) and 91,315 SNPs from PGC29 GWAS clumped at Pthreshold = 1 (c,d). Plotted values show the mean PRS Pleiotropy from 10-fold cross-validation in UKB.
Discussion
In this paper, we address the power–specificity tradeoff between deep and shallow MDD phenotypes by integrating them using phenotype imputation or MTAG. We show that the integrated MDD phenotypes greatly improve GWAS power and PRS accuracy while, crucially, preserving the genetic architecture of MDD. We propose a novel metric to assess the disorder specificity of a PRS that is widely applicable to biobank-based GWASs. This metric characterizes a power–specificity tradeoff. For MTAG, adding more phenotypes increases power but generally sacrifices specificity. Imputing LifetimeMDD with SoftImpute, on the other hand, better preserves specificity. Overall, both approaches to phenotype integration outperform either deep or shallow phenotypes alone.
Phenotype imputation is a simple and scalable approach that should be considered for most biobank-based genetic studies. An important but challenging future application will be longitudinal data, which are often sporadically measured across time and individuals with potentially extreme nonrandom missingness. One limitation of our specific imputation approaches is that they distort higher-order moments (Supplementary Fig. 1), which will bias some downstream analyses such as genetic correlation. In future work, this could be addressed with multiple imputation54 or with heteroskedasticity-aware downstream tests55.
MTAG has several important strengths that complement imputation. First, it operates on summary statistics, which enables incorporating external GWASs and is computationally cheap once GWASs have been performed. Second, we found that MTAG.All generally outperforms imputation in GWAS hits and PRS power and portability. However, MTAG generally has less specificity. A striking exception is MTAG.FamilyHistory, consistent with prior methods that specifically leverage family history to improve genetic studies44,45,56. However, MTAG is highly sensitive to input phenotype selection and therefore requires extensive domain knowledge, similar to other work combining multiple depression measures14. There are several extensions to further improve MTAG for phenotype integration. First, we could incorporate local estimates of genetic correlation in MTAG, which has improved LifetimeMDD PRSs in UK Biobank57. Second, we could directly combine PRSs for multiple traits using weights to optimize prediction58–60. Third, we could develop a more systematic approach to choosing MTAG inputs, but this search is limited by the computational cost of performing cross-validated GWASs on each considered trait. Fourth, parametric models of confounding in summary statistics, such as GWAS-by-subtraction61, could improve specificity. However, these models rely on choosing appropriate inputs and causal models, which is not straightforward for heterogeneous disorders. Finally, we could input the GWASs on imputed phenotypes to MTAG; however, this may exacerbate their biases because imputation inflates the correlations between traits that MTAG leverages (Supplementary Fig. 1).
Our study has implications for improving disorder specificity in future MDD studies. We have worked on the deepest MDD phenotype in UK Biobank, LifetimeMDD, which is derived by applying DSM-5 criteria in silico to self-rated MDD symptoms in the MHQ. Although it lies on essentially the same genetic liability continuum as gold-standard MDD11, LifetimeMDD is shallow compared to a clinical diagnosis based on a structured in-person interview due to self-report biases and misdiagnoses62–65. In the future, this bias could be mitigated using probability weights, which have recently been used for GWASs66,67. More broadly, the DSM-5 criteria for MDD have substantial shortcomings in reliability68–70. Nonetheless, improving the MDD diagnostic criteria may be only achievable through epistemic iterations64, a series of efforts to characterize specific genetic signals for the deepest available MDD definition and, in turn, refine our definition of MDD. Our efforts to improve GWAS power and specificity in noisy biobanks advance this process.
Our implementation of phenotype integration uses shallow MDD phenotypes to improve power for LifetimeMDD, and as such its specificity is limited by the specificity of LifetimeMDD. Future statistical methods could go further and improve specificity over existing phenotypes. We have taken a step in this direction with SoftImpute factors, which revealed a specific locus from a shallow phenotype. This is akin to latent factor correction in genomic studies23,71–74, and it could be adapted to AutoComplete using, for example, Integrated Gradients methods75. However, it is challenging to remove nonspecific signals without removing specific signals or, worse, introducing artificial signals due to collider bias23,42,76. This is especially true for disorders such as MDD where epistemic uncertainty clouds what signals are most biomedically useful.
Phenotype integration is broadly applicable to biobank-based genetic studies, which often evaluate a mixture of biomarker-, nurse-, GP-, specialist- and/or patient-defined disorder measures. Further, biobanks offer diverse disorder-relevant phenotypes, such as age of onset, medical procedures, prescriptions, environmental risk factors, family history and socioeconomic measures. While the degree of specificity of phenotype integration will vary between applications, this can be assessed with our novel PRS pleiotropy metric. This empirical measure complements prior theoretical derivations of power–specificity tradeoffs in the meta-analysis of heterogeneous traits77. In the future, PRS pleiotropy can be used to evaluate the specificity of newly constructed phenotypes.
Our results have complex implications for equity in genetic studies and clinical care. On the one hand, EHR-derived phenotypes have a history of exacerbating inequities that continues to this day78–81. Moreover, phenotype integration uses a reference phenome, which has the potential to propagate systematic biases present in biobank data. Although we found that phenotype integration can improve PRS portability across ancestries, the results are less statistically clear than those in European ancestries. This highlights the need for greater sample sizes in diverse ancestries and better methods for cross-ancestry portability. Careful extensions of our approach, such as residualizing phenome-wide factors, have the potential to improve portability by eliminating confounders. Given the extreme Eurocentric biases in available genomics data, these and other statistical approaches to improve the utility of PRSs for all people are urgently needed.
Methods
Ethical approval
This research was conducted under the ethical approval from the UK Biobank resource under application no. 28709 and 33217. The use of iPSYCH data follows the standards of the Danish Scientific Ethics Committee, the Danish Health Data Authority, the Danish Data Protection Agency and the Danish Neonatal Screening Biobank Steering Committee. Data access was via secure portals in accordance with Danish data protection guidelines set by the Danish Data Protection Agency, the Danish Health Data Authority and Statistics Denmark. Retrospective data collection and analysis for ATLAS was approved by the UCLA Institutional Review Board (IRB)48. Patient Recruitment and Sample Collection for Precision Health Activities at UCLA” is an approved study by the UCLA IRB (UCLA IRB17-001013). All necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived. The CONVERGE study was approved by the ethical review boards of Oxford University and participating hospitals. All participants provided written informed consent9.
Phenotypes used in phenotype imputation
We considered 217 relevant phenotypes to impute LifetimeMDD11 in 337,127 individuals of white British ancestry in UK Biobank (Supplementary Table 1). These included (1) LifetimeMDD as defined in Cai et al.11; (2) minimal phenotyping definitions of depression based on help-seeking, symptoms, self-reports and/or EHRs as defined in Cai et al.11; (3) individual lifetime and current MDD symptoms from the CIDI-SF82 and PHQ9 from which we derived LifetimeMDD; (4) psychosocial factors; (5) self-reported comorbidities; (6) family history of common diseases; (7) early life factors; (8) socioeconomic phenotypes; (9) lifestyle and environment phenotypes; (10) social support status; and (11) demographic features including age, sex, UK Biobank assessment center as a proxy for geographical residence and 20 genetic PCs. These phenotypes were selected based on their established relevance to MDD and were all collected through either a touchscreen questionnaire completed at the assessment center or the online mental health follow-up questionnaire (MHQ). All UK Biobank data fields, sample sizes and prevalence of binary outcomes are detailed in Supplementary Table 1, and we report levels of missingness for all inputs for multi-phenotype imputation in Extended Data Fig. 1. For PRS pleiotropy analyses, we excluded the 20 genetic PCs, 22 assessment centers and the genotyping array.
Phenotype imputation with SoftImpute
We fit SoftImpute with the ALS method24 on the 217 phenotypes making up the MDD-related phenome in UK Biobank, using cross-validation to optimize the nuclear norm regularization parameter. We used our prior approach to make the cross-validation more realistic by copying real missingness patterns instead of completely random entries17,83, which provides more realistic estimates of imputation accuracy (Extended Data Fig. 1). We previously studied SoftImpute at a smaller scale in comprehensive simulations and several real datasets17, and we have since used it in several larger studies17,83,84. Overall, SoftImpute is extremely simple, robust and scalable. We summarize the SoftImpute model fit by the latent factors (Fig. 3c) and the variances they explain (Fig. 3a), which are akin to the eigenvectors (or PCs) and eigenvalues of the phenotype covariance matrix, respectively. We also estimate the prediction strength (Fig. 3b), which is the squared correlation between two latent factors estimated after splitting the sample into two nonoverlapping halves (R2). We define the effective sample size after-imputation as Nobs + Nmiss ⨯ R2, analogous to genotype imputation17,18,25; we note that this approximates the power-equivalent number of observed phenotypes.
Phenotype imputation with AutoComplete
We developed a new deep learning-based method, AutoComplete, in a companion paper29. AutoComplete consists of several fully connected layers with nonlinearities and learns to optimize reconstruction of realistically held-out missing entries. The model is fully differentiable and is fit using stochastic gradient descent. Unlike SoftImpute, AutoComplete’s objective function models binary phenotypes. As with SoftImpute, the hyperparameters for AutoComplete were determined through cross-validation on realistically held-out missing data. In this paper, we focus on its application to imputing LifetimeMDD.
GWASs on observed or imputed phenotypes
GWASs on directly phenotyped and imputed phenotypes in UK Biobank were performed using imputed genotype data at 5,781,354 SNPs (minor allele frequency > 5%, INFO score > 0.9) using logistic regression and linear regression implemented in PLINK v2 (ref. 85) for binary and quantitative traits, respectively. We used 20 PCs computed with flashPCA86 on 337,129 white British individuals in UK Biobank and genotyping arrays as covariates for all GWASs (see Supplementary Note for details of sample and genotype quality control in UK Biobank). To test for heterogeneity between genetic effects found in GWASs on observed LifetimeMDD and imputed measures of MDD from SoftImpute (Soft-ImpOnly) and AutoComplete (Auto-ImpOnly), we performed a random-effect meta-analysis using METASOFT33 and tested for heterogeneity between effect sizes at each SNP.
SNP-based heritability and genetic correlation
To test for SNP-based heritability of each phenotype and the genetic correlation between pairs of phenotypes, linkage disequilibrium (LD) score regression implemented in LDSC v1.0.11 (refs. 32,87) was performed on the GWAS summary statistics using in-sample LD scores estimated in 10,000 random white British UK Biobank individuals at SNPs with minor allele frequency > 5% as reference. For MTAG results, we used the effective sample size estimated in MTAG as the sample size entry in LDSC; for all other GWASs, we used the actual sample size. When we estimated the liability-scale heritability, we assumed the population prevalence of binary phenotypes equaled their prevalence in UK Biobank. For all GWASs, we have indicated their effective sample sizes accounting for imbalance between cases and controls (neffective = 4/(1/ncases + 1/ncontrols)) in Supplementary Tables 5 and 6. We note that this is different from the imputation-related definition of effective sample size and also differs from MTAG’s definition.
In-sample PRS prediction of phenotypes in UK Biobank with ten-fold cross-validation
We performed SoftImpute24 and AutoComplete imputations ten times. Each time we used 90% of the individuals in the input phenotype matrix and then built the PRS from the GWAS results with PRSice v2 (ref. 88) and evaluated predictive accuracy for observed LifetimeMDD and the depression-related phenome (217 phenotypes, used as input in imputation) in the 10% of individuals who were held out. For MTAG19, we performed a GWAS on each set of input phenotypes (as shown in Fig. 4) ten times, each time using 90% of the individuals in UK Biobank. We then ran MTAG on the GWAS summary statistics on this 90%, built a PRS from the resulting MTAG summary statistics with PRSice v2 and evaluated predictive accuracy for observed LifetimeMDD in the 10% of individuals who were held out. For all PRS predictions, we used 20 genomic PCs and the genotyping array used as covariates. For binary phenotypes, including LifetimeMDD, we evaluated accuracy using Nagelkerke’s R2. For all quantitative phenotypes, including neuroticism, we evaluated accuracy using ordinary R2.
PRS prediction of phenotypes in UK Biobank from external GWAS summary statistics
We constructed PRSs from MDD GWAS summary statistics from PGC29 (ref. 4), iPSYCH15 and 23andMe7, as detailed in Supplementary Table 6, and predicted phenotypes in UK Biobank using PRSice v2, with 20 genomic PCs and the genotyping array used in UK Biobank as covariates. For each of these studies, we used only SNPs with imputation INFO score > 0.9 and minor allele frequency > 5% for constructing the PRS. For binary phenotypes, including LifetimeMDD, we evaluated accuracy using Nagelkerke’s R2. For all quantitative phenotypes, including neuroticism, we evaluated accuracy using ordinary R2. We calculated PRS pleiotropy on all secondary phenotypes (not including LifetimeMDD) used in imputation, except for 20 PCs, array and assessment center (total of 172 phenotypes).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41588-023-01559-9.
Supplementary information
Supplementary Note, Figs.1–11, legends to Tables 1–8 and references.
Acknowledgements
A.D., S.-A.B., J.F., A.J.S., S.S., K.S.K. and N.C. are supported by R01MH130581 from the NIH. M.T. is supported in part by the NIH Training Grant in Genomic Analysis and Interpretation T32HG002536. U.A. and S.S. are supported in part by III-1705121, CAREER-1943497 and R35GM125055. V.A. is supported by Lundbeck Foundation postdoctoral grant R380-2021-1465. A.J.S. is supported by Lundbeckfonden Fellowship R335-2019-2318. R.B. is supported by T32-NS048004 from the NIH. The iPSYCH team was supported by grants from the Lundbeck Foundation (R102-A9118, R155-2014-1724 and R248-2017-2003), NIMH (1R01MH124851-01) and the Universities and University Hospitals of Aarhus and Copenhagen. The Danish National Biobank resource was supported by the Novo Nordisk Foundation. High-performance computer capacity for handling and statistical analysis of iPSYCH data on the GenomeDK HPC facility was provided by the Center for Genomics and Personalized Medicine and the Centre for Integrative Sequencing, iSEQ, Aarhus University, Denmark. We gratefully acknowledge the support of all collaborators and participants in UK Biobank, iPSYCH, CONVERGE and UCLA ATLAS who made this work possible.
Extended data
Author contributions
A.D. and N.C. wrote the paper. A.D., J.F., K.S.K. and N.C. designed the study. M.T. performed ATLAS analyses. U.A. and S.S. performed AutoComplete imputation. A.D. and N.C. performed all other analyses. M.K., V.A., T.W. and A.J.S. supported iPSYCH analyses. R.B. and S.-A.B. edited the manuscript. All authors reviewed the paper.
Peer review
Peer review information
Nature Genetics thanks Andrew McIntosh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Funding
Open access funding provided by Helmholtz Zentrum München - Deutsches Forschungszentrum für Gesundheit und Umwelt (GmbH).
Data availability
UK Biobank genotype and phenotype data used in this study are from the full release (imputation version 2) of the UK Biobank Resource obtained under application no. 28709 and 33217. We used publicly available summary statistics from PGC29 and 23andMe from the Psychiatric Genomics Consortium (https://www.med.unc.edu/pgc/results-and-downloads), as well as summary statistics for affective disorders in the iPSYCH2012 cohort (10.6084/m9.figshare.20517330), with references in Supplementary Table 4. The individual-level CONVERGE, Danish and UCLA datasets are not publicly available due to institutional restrictions on data sharing and privacy concerns. Summary statistics of all GWASs used in this paper are available at: 10.6084/m9.figshare.19604335. PRSs for all imputed and MTAG GWASs in this study have been submitted to the PGS Catalogue under publication ID PGP000461 and have been assigned score IDs from PGS003576 to PGS003585.
Code availability
Publicly available tools that were used in data analyses are described wherever relevant in the Methods and Reporting Summary. Custom code for SoftImpute imputation of the MDD-relevant phenome and calculating PRS pleiotropy is available at https://github.com/andywdahl/mdd-impute and https://github.com/caina89/MDDImpute. The AutoComplete software is available at https://github.com/sriramlab/AutoComplete.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Andrew Dahl, Na Cai.
Contributor Information
Andrew Dahl, Email: andywdahl@uchicago.edu.
Na Cai, Email: na.cai@helmholtz-munich.de.
Extended data
is available for this paper at 10.1038/s41588-023-01559-9.
Supplementary information
The online version contains supplementary material available at 10.1038/s41588-023-01559-9.
References
- 1.Flint J, Kendler KS. The genetics of major depression. Neuron. 2014;81:484–503. doi: 10.1016/j.neuron.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flint J. The genetic basis of major depressive disorder. Mol. Psychiatry. 2023 doi: 10.1038/s41380-023-01957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 4.Wray NR, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018;50:668–681. doi: 10.1038/s41588-018-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howard DM, et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 2018;9:1470. doi: 10.1038/s41467-018-03819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard DM, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 2019;22:343–352. doi: 10.1038/s41593-018-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyde CL, et al. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat. Genet. 2016;48:1031–1036. doi: 10.1038/ng.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey DF, et al. Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in >1.2 million individuals highlight new therapeutic directions. Nat. Neurosci. 2021;24:954–963. doi: 10.1038/s41593-021-00860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CONVERGE consortium. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature523, 588–591 (2015). [DOI] [PMC free article] [PubMed]
- 10.McIntosh AM, Sullivan PF, Lewis CM. Uncovering the genetic architecture of major depression. Neuron. 2019;102:91–103. doi: 10.1016/j.neuron.2019.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai N, et al. Minimal phenotyping yields genome-wide association signals of low specificity for major depression. Nat. Genet. 2020;52:437–447. doi: 10.1038/s41588-020-0594-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell BL, et al. Polygenic risk scores derived from varying definitions of depression and risk of depression. JAMA Psychiatry. 2021;78:1152–1160. doi: 10.1001/jamapsychiatry.2021.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jermy BS, Glanville KP, Coleman JRI, Lewis CM, Vassos E. Exploring the genetic heterogeneity in major depression across diagnostic criteria. Mol. Psychiatry. 2021;26:7337–7345. doi: 10.1038/s41380-021-01231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glanville KP, et al. Multiple measures of depression to enhance validity of major depressive disorder in the UK Biobank. BJPsych Open. 2021;7:e44. doi: 10.1192/bjo.2020.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schork AJ, et al. A genome-wide association study of shared risk across psychiatric disorders implicates gene regulation during fetal neurodevelopment. Nat. Neurosci. 2019;22:353–361. doi: 10.1038/s41593-018-0320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bycroft C, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahl A, et al. A multiple-phenotype imputation method for genetic studies. Nat. Genet. 2016;48:466–472. doi: 10.1038/ng.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hormozdiari F, et al. Imputing phenotypes for genome-wide association studies. Am. J. Hum. Genet. 2016;99:89–103. doi: 10.1016/j.ajhg.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turley P, et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat. Genet. 2018;50:229–237. doi: 10.1038/s41588-017-0009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leek JT, Storey JD. Capturing heterogeneity in gene expression studies by surrogate variable analysis. PLoS Genet. 2007;3:1724–1735. doi: 10.1371/journal.pgen.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stegle O, Parts L, Piipari M, Winn J, Durbin R. Using probabilistic estimation of expression residuals (PEER) to obtain increased power and interpretability of gene expression analyses. Nat. Protoc. 2012;7:500–507. doi: 10.1038/nprot.2011.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GTEx Consortium et al. Genetic effects on gene expression across human tissues. Nature550, 204–213 (2017). [DOI] [PMC free article] [PubMed]
- 23.Dahl A, Guillemot V, Mefford J, Aschard H, Zaitlen N. Adjusting for principal components of molecular phenotypes induces replicating false positives. Genetics. 2019;211:1179–1189. doi: 10.1534/genetics.118.301768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazumder R, Hastie T, Tibshirani R. Spectral regularization algorithms for learning large incomplete matrices. J. Mach. Learn. Res. 2010;11:2287–2322. [PMC free article] [PubMed] [Google Scholar]
- 25.Marchini J, Howie B. Genotype imputation for genome-wide association studies. Nat. Rev. Genet. 2010;11:499–511. doi: 10.1038/nrg2796. [DOI] [PubMed] [Google Scholar]
- 26.Kendler KS, Gardner CO, Neale MC, Prescott CA. Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychol. Med. 2001;31:605–616. doi: 10.1017/S0033291701003907. [DOI] [PubMed] [Google Scholar]
- 27.Kendler KS, Ohlsson H, Lichtenstein P, Sundquist J, Sundquist K. The genetic epidemiology of treated major depression in Sweden. Am. J. Psychiatry. 2018;175:1137–1144. doi: 10.1176/appi.ajp.2018.17111251. [DOI] [PubMed] [Google Scholar]
- 28.Kendler KS, Gardner CO. Sex differences in the pathways to major depression: a study of opposite-sex twin pairs. Am. J. Psychiatry. 2014;171:426–435. doi: 10.1176/appi.ajp.2013.13101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.An, U. et al. Deep learning-based phenotype imputation on population-scale biobank data increases genetic discoveries. Nat. Genet.10.1038/s41588-023-01558-w (2023). [DOI] [PMC free article] [PubMed]
- 30.Pedersen CB, et al. The iPSYCH2012 case-cohort sample: new directions for unravelling genetic and environmental architectures of severe mental disorders. Mol. Psychiatry. 2018;23:6–14. doi: 10.1038/mp.2017.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kendler KS, et al. Shared and specific genetic risk factors for lifetime major depression, depressive symptoms and neuroticism in three population-based twin samples. Psychol. Med. 2019;49:2745–2753. doi: 10.1017/S003329171800377X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bulik-Sullivan BK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015;47:291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han B, Eskin E. Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. Am. J. Hum. Genet. 2011;88:586–598. doi: 10.1016/j.ajhg.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tibshirani R, Walther G. Cluster validation by prediction strength. J. Comput. Graph. Stat. 2005;14:511–528. doi: 10.1198/106186005X59243. [DOI] [Google Scholar]
- 35.Nagel M, et al. Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat. Genet. 2018;50:920–927. doi: 10.1038/s41588-018-0151-7. [DOI] [PubMed] [Google Scholar]
- 36.Baselmans BML, et al. Multivariate genome-wide analyses of the well-being spectrum. Nat. Genet. 2019;51:445–451. doi: 10.1038/s41588-018-0320-8. [DOI] [PubMed] [Google Scholar]
- 37.Yao X, et al. Integrative analysis of genome-wide association studies identifies novel loci associated with neuropsychiatric disorders. Transl. Psychiatry. 2021;11:69. doi: 10.1038/s41398-020-01195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Z, et al. Shared genetic and experimental links between obesity-related traits and asthma subtypes in UK Biobank. J. Allergy Clin. Immunol. 2020;145:537–549. doi: 10.1016/j.jaci.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pisanu C, et al. Evidence that genes involved in hedgehog signaling are associated with both bipolar disorder and high BMI. Transl. Psychiatry. 2019;9:315. doi: 10.1038/s41398-019-0652-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winkler TW, et al. The influence of age and sex on genetic associations with adult body size and shape: a large-scale genome-wide interaction study. PLoS Genet. 2015;11:e1005378. doi: 10.1371/journal.pgen.1005378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann TJ, et al. A large multiethnic genome-wide association study of adult body mass index identifies novel loci. Genetics. 2018;210:499–515. doi: 10.1534/genetics.118.301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aschard H, Vilhjálmsson BJ, Joshi AD, Price AL, Kraft P. Adjusting for heritable covariates can bias effect estimates in genome-wide association studies. Am. J. Hum. Genet. 2015;96:329–339. doi: 10.1016/j.ajhg.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Day FR, Loh P-R, Scott RA, Ong KK, Perry JRB. A robust example of collider bias in a genetic association study. Am. J. Hum. Genet. 2016;98:392–393. doi: 10.1016/j.ajhg.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu JZ, Erlich Y, Pickrell JK. Case-control association mapping by proxy using family history of disease. Nat. Genet. 2017;49:325–331. doi: 10.1038/ng.3766. [DOI] [PubMed] [Google Scholar]
- 45.Hujoel MLA, Gazal S, Loh P-R, Patterson N, Price AL. Liability threshold modeling of case-control status and family history of disease increases association power. Nat. Genet. 2020;52:541–547. doi: 10.1038/s41588-020-0613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill WD, et al. A combined analysis of genetically correlated traits identifies 187 loci and a role for neurogenesis and myelination in intelligence. Mol. Psychiatry. 2019;24:169–181. doi: 10.1038/s41380-017-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bybjerg-Grauholm, J. et al. The iPSYCH2015 Case-Cohort sample: updated directions for unravelling genetic and environmental architectures of severe mental disorders. Preprint at medRxiv10.1101/2020.11.30.20237768 (2020). [DOI] [PMC free article] [PubMed]
- 48.Johnson R, et al. Leveraging genomic diversity for discovery in an electronic health record linked biobank: the UCLA ATLAS Community Health Initiative. Genome Med. 2022;14:104. doi: 10.1186/s13073-022-01106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson R, et al. The UCLA ATLAS Community Health Initiative: promoting precision health research in a diverse biobank. Cell Genom. 2023;3:1. doi: 10.1016/j.xgen.2022.100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peterson RE, et al. The genetic architecture of major depressive disorder in Han Chinese women. JAMA Psychiatry. 2017;74:162–168. doi: 10.1001/jamapsychiatry.2016.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin AR, et al. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 2019;51:584–591. doi: 10.1038/s41588-019-0379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin AR, et al. Human demographic history impacts genetic risk prediction across diverse populations. Am. J. Hum. Genet. 2017;100:635–649. doi: 10.1016/j.ajhg.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterson RE, et al. Genome-wide association studies in ancestrally diverse populations: opportunities, methods, pitfalls, and recommendations. Cell. 2019;179:589–603. doi: 10.1016/j.cell.2019.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Little, R. J. A. & Rubin, D. B. Statistical Analysis With Missing Data: Little/Statistical Analysis With Missing Data (John Wiley & Sons 2002).
- 55.McCaw, Z. R., Gao, J., Lin, X. & Gronsbell, J. Leveraging a machine learning derived surrogate phenotype to improve power for genome-wide association studies of partially missing phenotypes in population biobanks. Preprint at bioRxiv10.1101/2022.12.12.520180 (2022).
- 56.Pedersen EM, et al. Accounting for age of onset and family history improves power in genome-wide association studies. Am. J. Hum. Genet. 2022;109:417–432. doi: 10.1016/j.ajhg.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pain, O. & Lewis, C. M. Using local genetic correlation improves polygenic score prediction across traits. Preprint at bioRxiv10.1101/2022.03.10.483736 (2022).
- 58.Krapohl E, et al. Multi-polygenic score approach to trait prediction. Mol. Psychiatry. 2018;23:1368–1374. doi: 10.1038/mp.2017.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pain O, et al. Evaluation of polygenic prediction methodology within a reference-standardized framework. PLoS Genet. 2021;17:e1009021. doi: 10.1371/journal.pgen.1009021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung W, et al. Efficient cross-trait penalized regression increases prediction accuracy in large cohorts using secondary phenotypes. Nat. Commun. 2019;10:569. doi: 10.1038/s41467-019-08535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Demange PA, et al. Investigating the genetic architecture of noncognitive skills using GWAS-by-subtraction. Nat. Genet. 2021;53:35–44. doi: 10.1038/s41588-020-00754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uher R, et al. Self-report and clinician-rated measures of depression severity: can one replace the other? Depress. Anxiety. 2012;29:1043–1049. doi: 10.1002/da.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cuijpers P, Li J, Hofmann SG, Andersson G. Self-reported versus clinician-rated symptoms of depression as outcome measures in psychotherapy research on depression: a meta-analysis. Clin. Psychol. Rev. 2010;30:768–778. doi: 10.1016/j.cpr.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 64.Fried EI, Flake JK, Robinaugh DJ. Revisiting the theoretical and methodological foundations of depression measurement. Nat. Rev. Psychol. 2022;1:358–368. doi: 10.1038/s44159-022-00050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adams MJ, et al. Factors associated with sharing e-mail information and mental health survey participation in large population cohorts. Int. J. Epidemiol. 2020;49:410–421. doi: 10.1093/ije/dyz134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Alten, S., Domingue, B. W., Galama, T. & Marees, A. T. Reweighting the UK Biobank to reflect its underlying sampling population substantially reduces pervasive selection bias due to volunteering. Preprint at bioRxiv10.1101/2022.05.16.22275048 (2022)
- 67.Schoeler T, et al. Participation bias in the UK Biobank distorts genetic associations and downstream analyses. Nat. Hum. Behav. 2023;7:1216–1227. doi: 10.1038/s41562-023-01579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schatzberg AF. Scientific issues relevant to improving the diagnosis, risk assessment, and treatment of major depression. Am. J. Psychiatry. 2019;176:342–347. doi: 10.1176/appi.ajp.2019.19030273. [DOI] [PubMed] [Google Scholar]
- 69.Regier DA, et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am. J. Psychiatry. 2013;170:59–70. doi: 10.1176/appi.ajp.2012.12070999. [DOI] [PubMed] [Google Scholar]
- 70.Freedman R, et al. The initial field trials of DSM-5: new blooms and old thorns. Am. J. Psychiatry. 2013;170:1–5. doi: 10.1176/appi.ajp.2012.12091189. [DOI] [PubMed] [Google Scholar]
- 71.Kang HM, Ye C, Eskin E. Accurate discovery of expression quantitative trait loci under confounding from spurious and genuine regulatory hotspots. Genetics. 2008;180:1909–1925. doi: 10.1534/genetics.108.094201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joo JWJ, Sul JH, Han B, Ye C, Eskin E. Effectively identifying regulatory hotspots while capturing expression heterogeneity in gene expression studies. Genome Biol. 2014;15:r61. doi: 10.1186/gb-2014-15-4-r61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brynedal B, et al. Large-scale trans-eQTLs affect hundreds of transcripts and mediate patterns of transcriptional co-regulation. Am. J. Hum. Genet. 2017;100:581–591. doi: 10.1016/j.ajhg.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yao C, et al. Dynamic role of trans regulation of gene expression in relation to complex traits. Am. J. Hum. Genet. 2017;100:571–580. doi: 10.1016/j.ajhg.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sundararajan, M., Taly, A. & Yan, Q. Axiomatic attribution for deep networks. Preprint at arXiv10.48550/ARXIV.1703.01365 (2017).
- 76.Aschard H, et al. Covariate selection for association screening in multiphenotype genetic studies. Nat. Genet. 2017;49:1789–1795. doi: 10.1038/ng.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okbay A, et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat. Genet. 2016;48:624–633. doi: 10.1038/ng.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hsu C-Y, et al. Race, genetic ancestry, and estimating kidney function in CKD. N. Engl. J. Med. 2021;385:1750–1760. doi: 10.1056/NEJMoa2103753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang Y, et al. Polygenic transcriptome risk scores (PTRS) can improve portability of polygenic risk scores across ancestries. Genome Biol. 2022;23:23. doi: 10.1186/s13059-021-02591-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amariuta T, et al. Improving the trans-ancestry portability of polygenic risk scores by prioritizing variants in predicted cell-type-specific regulatory elements. Nat. Genet. 2020;52:1346–1354. doi: 10.1038/s41588-020-00740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weissbrod O, et al. Leveraging fine-mapping and multipopulation training data to improve cross-population polygenic risk scores. Nat. Genet. 2022;54:450–458. doi: 10.1038/s41588-022-01036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davis KAS, et al. Mental health in UK Biobank — development, implementation and results from an online questionnaire completed by 157 366 participants: a reanalysis. BJPsych Open. 2020;6:e18. doi: 10.1192/bjo.2019.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dahl A, et al. A robust method uncovers significant context-specific heritability in diverse complex traits. Am. J. Hum. Genet. 2020;106:71–91. doi: 10.1016/j.ajhg.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dahl A, et al. Reverse GWAS: using genetics to identify and model phenotypic subtypes. PLoS Genet. 2019;15:e1008009. doi: 10.1371/journal.pgen.1008009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang CC, et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abraham G, Inouye M. Fast principal component analysis of large-scale genome-wide data. PLoS ONE. 2014;9:e93766. doi: 10.1371/journal.pone.0093766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bulik-Sullivan B, et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 2015;47:1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi SW, O’Reilly PF. PRSice-2: Polygenic Risk Score software for biobank-scale data. Gigascience. 2019;8:giz082. doi: 10.1093/gigascience/giz082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Note, Figs.1–11, legends to Tables 1–8 and references.
Data Availability Statement
UK Biobank genotype and phenotype data used in this study are from the full release (imputation version 2) of the UK Biobank Resource obtained under application no. 28709 and 33217. We used publicly available summary statistics from PGC29 and 23andMe from the Psychiatric Genomics Consortium (https://www.med.unc.edu/pgc/results-and-downloads), as well as summary statistics for affective disorders in the iPSYCH2012 cohort (10.6084/m9.figshare.20517330), with references in Supplementary Table 4. The individual-level CONVERGE, Danish and UCLA datasets are not publicly available due to institutional restrictions on data sharing and privacy concerns. Summary statistics of all GWASs used in this paper are available at: 10.6084/m9.figshare.19604335. PRSs for all imputed and MTAG GWASs in this study have been submitted to the PGS Catalogue under publication ID PGP000461 and have been assigned score IDs from PGS003576 to PGS003585.
Publicly available tools that were used in data analyses are described wherever relevant in the Methods and Reporting Summary. Custom code for SoftImpute imputation of the MDD-relevant phenome and calculating PRS pleiotropy is available at https://github.com/andywdahl/mdd-impute and https://github.com/caina89/MDDImpute. The AutoComplete software is available at https://github.com/sriramlab/AutoComplete.