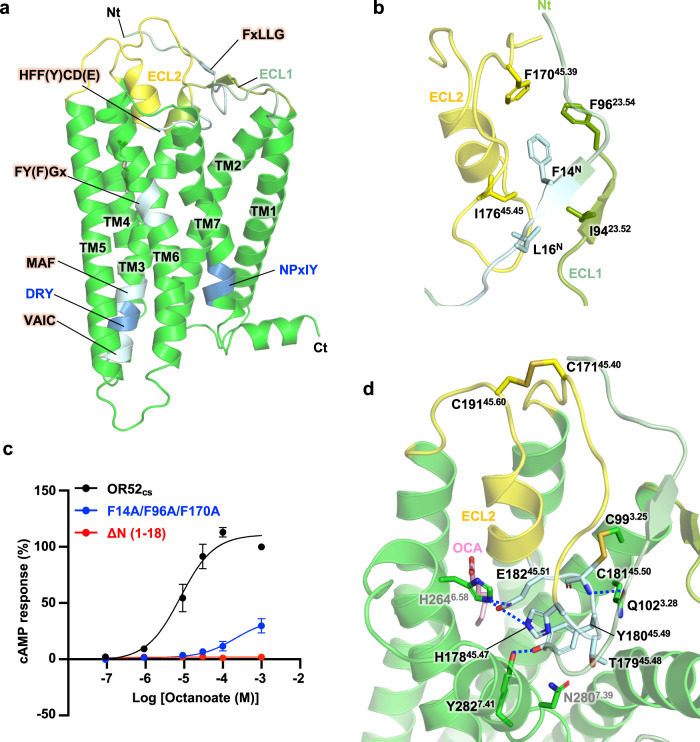
Fig. 2. Structural features of OR52cs.
a Overall structure of OR52cs highlighting conserved motifs. The OR-specific conserved motifs are colored in light blue, and the conventional motifs present in class A GPCRs (DRY and NPxIY motifs) are colored in blue. N-tail, ECL1, and ECL2 are colored in pale green, split pea, and yellow, respectively. The TM helices and structural motifs are labeled. b Detailed interactions among the N-tail, ECL1, and ECL2. Residues interacting with F14N and L16N in the conserved FxLLG motif are shown as sticks. c Dose-dependent cAMP response curves of the N-tail deletion mutant (ΔN(1–18)) and the F14A/F96A/F170A mutant. Each data point represents the mean ± standard error of the mean (S.E.M.) from n = 3 independent experiments. d Interactions of ECL2 with TMs 3, 6, and 7. The two disulfide bonds, C993.25-C18145.50 and C17145.40-C19145.60, are shown as sticks. Residues in the conserved HTYCE motif are colored in light blue. Highly conserved residues in ORs are labeled in black, whereas less conserved residues are labeled in gray.