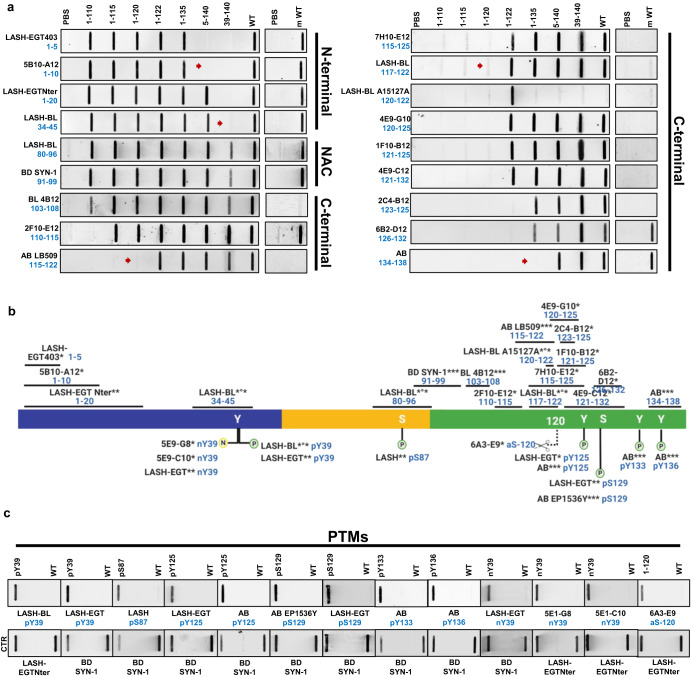
Fig. 2. Validation and epitope mapping of aSyn antibodies.
a DB validation of the novel monoclonal, in-house polyclonal and commercially available aSyn antibodies against the N-terminal, NAC and the C-terminal regions of aSyn for epitope mapping, specificity and species reactivity using a selected library of aSyn recombinant proteins under native conditions. Protein loading control was run via Ponceau S staining. All loaded proteins represent human aSyn forms except for mouse aSyn wild-type (m WT) protein. Red arrows highlight the sensitivities of the antibodies to the presence of neighboring aSyn PTMs. b A schematic to represent the novel monoclonal (marked with *), in-house polyclonal (marked with **) and pre-existing commercial aSyn antibodies (marked with ***) included in this study. The commercial antibodies developed jointly with Biolegend are marked with *°*. The epitope information of each antibody is indicated in blue. Schematic created with BioRender.com (agreement no: JR23G6G5LA). c Specificity validation of the aSyn PTM antibodies via DB screening. aSyn alpha-synuclein, CTR control, DB dot/slot blot, FL full-length, m mouse, NAC non-amyloid component, PBS phosphate-buffered saline, PTM post-translational modification, WT wild-type.