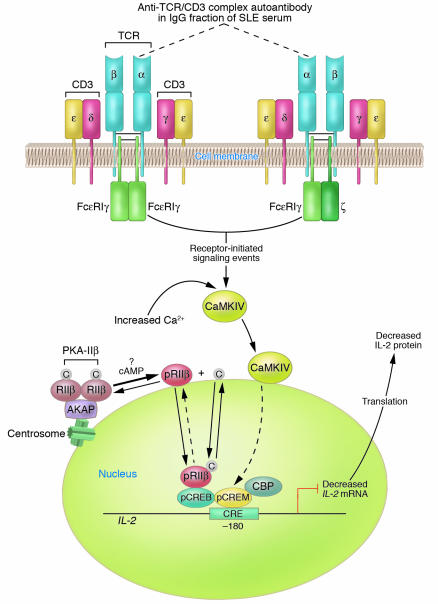
Figure 1.
Diagram of established and proposed mechanisms contributing to reduced IL-2 production by SLE T cells. A primary T cell disorder characterized by multiple abnormal signaling molecules has been identified in human SLE. A proportion of TCR ζ chains are replaced with FcεRIγ chains through which signaling can take place. In the microenvironment, anti-TCR/CD3 autoantibody(s) can bind to SLE T cells via the TCR/CD3 complex; however, the subunit(s) of the TCR/CD3 complex against which the autoantibody(s) is directed has not yet been identified. For the purpose of this illustration, the autoantibody is proposed to be against the α subunit of the TCR (dotted lines). Autoantibody binding to the TCR/CD3 complex results in heightened intracellular Ca2+ concentrations and activation of the CaMKIV signaling cascade. CaMKIV translocates to the nucleus, is proposed to phosphorylate CREM, and induces increased pCREM binding to the CRE located at the –180 region of the IL-2 promoter/enhancer. Because there is overexpression of the RIIβ in the T cell nuclei of some patients with SLE, it is proposed that PKA-IIβ is spontaneously activated by an unknown mechanism and that RIIβ becomes autophosphorylated; pRIIβ translocates to the nucleus, where it mediates downregulation of IL-2 transcription and IL-2 production. Together, pCREM and pRIIβ inhibit IL-2 transcription, thus yielding reduced IL-2 production. Note that proposed mechanisms have not been experimentally demonstrated. C, catalytic subunit; CBP, CREB binding protein.