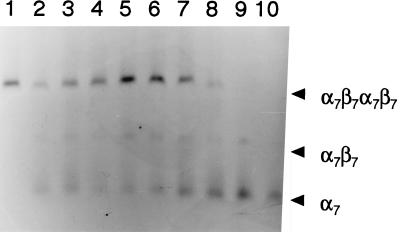
FIG. 5.
Temperature-dependent assembly of the M. thermophila 20S proteasome. The purified 20S proteasome produced in E. coli is shown in lane 1. Purified α7 rings and β prosubunits independently produced in E. coli were combined in subunit equimolar amounts (15 μM) and incubated for 60 min at 16°C (lane 2), 21°C (lane 3), 30°C (lane 4), 37°C (lane 5), 42°C (lane 6), 50°C (lane 7), 55°C (lane 8), or 60°C (lane 9). Purified α7 rings produced in E. coli independently of β prosubunits are in lane 10. Samples (1.1 μg) were analyzed by nondenaturing electrophoresis using 2.0% agarose. Proteins were stained with Coomassie blue R-250. The α7β7β7α7 20S proteasome, a putative α7β7 structure, and α7 rings are indicated by arrowheads (right). The unincorporated β prosubunits eluted from the gel and were not detectable.