Table 1.
Reaction Optimization
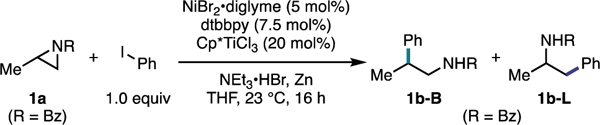
| ||||
|---|---|---|---|---|
| entry | deviation from standard conditionsa | aziridine conversion [%]b | yield [%]b | B:Lb |
| 1 | none | 100 | 81 | 12:1 |
|
| ||||
| 2 | R = Ac | 100 | 31 | 1:6 |
| 3 | R = Boc | 96 | 33 | 1:3 |
| 4 | R = Cbz | 100 | 13 | 1:1 |
|
| ||||
| 5 | CpTiCl3 | 88 | 40 | 3:1 |
| 6 | TMSCl instead of Ti | 36 | < 5 | – |
| 7 | TiCl4•THF2 | 80 | 24 | 1:1 |
| 8 | Cp2TiCl2 | 47 | <5 | – |
| 9 | (Cp*)2TiCl2 | 34 | <5 | – |
|
| ||||
| 10 | Mn | 93 | 71 | 11:1 |
| 11 | TDAE | 100 | 81 | 11:1 |
|
| ||||
| 12 | without Ni/ligand | 100 | 0 | – |
| 13 | without ligand | 100 | 9 | 11:1 |
| 14 | without Cp*TiCl3 | 32 | <5 | – |
| 15 | wtihout NEt3•HBr | 100 | 34 | 9:1 |
| 16 | without Zn | 86 | 0 | – |
|
| ||||
| ||||
NEt3•HBr (2.0 equiv), Zn (3.0 equiv), THF (0.15 M).
Reactions performed on 0.1 mmol scale. Yields and selectivity were determined by GC-FID with dodecane as an internal standard.
