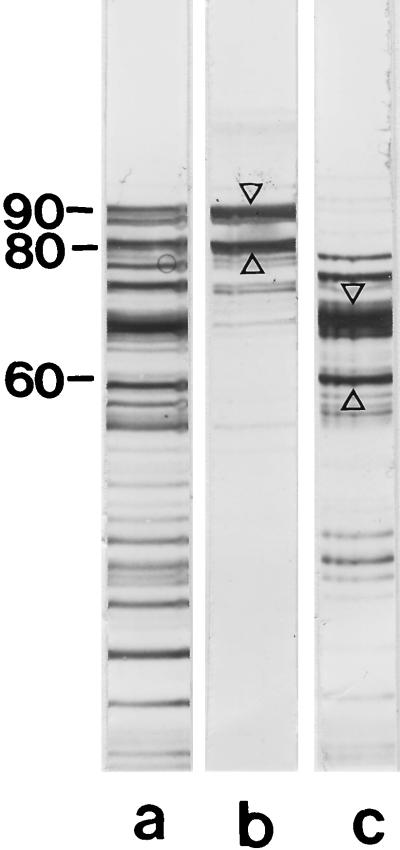
FIG. 3.
(a) SDS-PAGE pattern of the S-layer protein from B. stearothermophilus PV72/p6 after proteolytic degradation with endoproteinase Glu-C in presence of 2 M GHCl, dialysis against distilled water, and removal of insoluble material by centrifugation. Only two high-molecular-weight cleavage fragments with apparent molecular weights of 90,000 and 80,000 recognized native peptidoglycan-containing sacculi as binding sites and were detected in the pellet (lane b), while the lower-molecular-weight cleavage fragments remained in the clear supernatant (lane c). Protein bands which were subjected to N-terminal sequencing are indicated by arrow. Molecular weights are given in thousands.