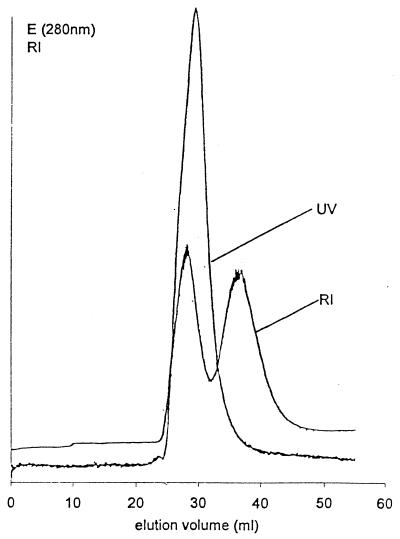
FIG. 4.
Elution profile of a mixture of proteolytic cleavage fragments from the S-layer protein from B. stearothermophilus PV72/p6 which had retained the affinity to bind to native peptidoglycan-containing sacculi (Fig. 3, lane b) and the isolated secondary cell wall polymer. Fractions representing the first peak contained comparable amounts of the proteolytic cleavage fragments with apparent molecular weights of 80,000 and 90,000 on SDS-gels. The second peak represented unbound secondary cell wall polymer.