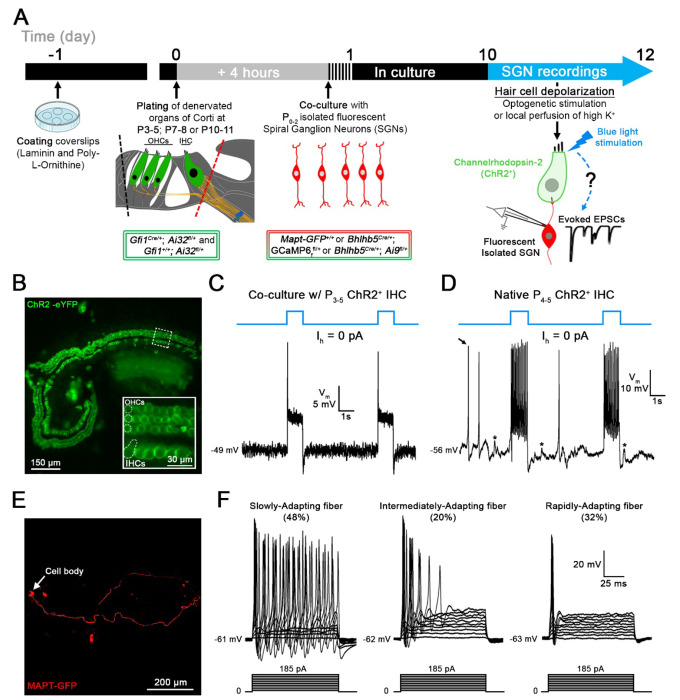
Figure 1. Co-cultures of denervated organs of Corti and isolated spiral ganglion neurons (SGNs) for testing regenerated hair cell synaptic function.
A, Organs of Corti were dissected from Gfi1Cre/+; Ai32fl/+ mice expressing Channelrhodopsin-2 (ChR2) in both inner hair cells (IHCs) and outer hair cells (OHCs) for light stimulation and from Gfi1+/+; Ai32fl/+ mice, where hair cell stimulation was performed by the local perfusion of 40 mM K+. Organs of Corti were separated from the lateral wall (black dotted line) and denervated by cutting through SGN endings close to the IHCs, (red dotted line). Denervated organs of Corti were plated at 3 ages, postnatal days (P)3-5, P7-8 or P10-11. Fluorescent SGNs were isolated at P0-2 from one of three mouse lines used indiscriminately (Mapt-GFP+/+; Bhlhb5Cre/+; GCaMP6ffl+ or Bhlhb5Cre/+; Ai9fl/+) and co-cultured with denervated organs of Corti. At 10 to 12 days in vitro (DIV), recordings were performed from SGN somata while stimulating hair cells. Regenerated functional synapses were identified by excitatory postsynaptic currents (EPSCs) in SGNs in response to IHC stimulation. B, Confocal image of a denervated P3-5 Gfi1Cre/+; Ai32fl/+ organ of Corti. Inset: ChR2 is observed in IHC and OHC membranes via eYFP tag co-expressed with ChR2. C, In current clamp mode, Iholding = 0 pA, the IHC membrane potential is recorded in response to two 1s blue light pulses separated by a 4s interval (blue line). Resting membrane potential indicated at the trace. The IHC response includes an initial peak, followed by a steady-state depolarization. D, Same protocol as in C. IHC recording in a native P4-5 Gfi1Cre/+; Ai32fl/+ acutely isolated organ of Corti. Light stimulation induces IHC depolarization and superimposed Ca2+ APs which are not found in cultured IHCs like in C. EPSPs (asterisks) and Ca2+ APs (black arrow) also occur spontaneously. E, Confocal image of a cultured MAPT-GFP positive SGN with soma and projecting fiber. F, SGNs with diverse electrical response properties in culture; ‘Slowly-Adapting’: APs slightly decrease in size throughout the pulse; ‘Intermediately-Adapting’: multiple APs only at the beginning of the pulse; ‘Rapidly-Adapting’: single AP at the pulse onset. Current clamp recordings of SGNs; 100ms long current step protocols from resting membrane potential as indicated at trace, with initial 5mV step and subsequent 10mV increasing steps.