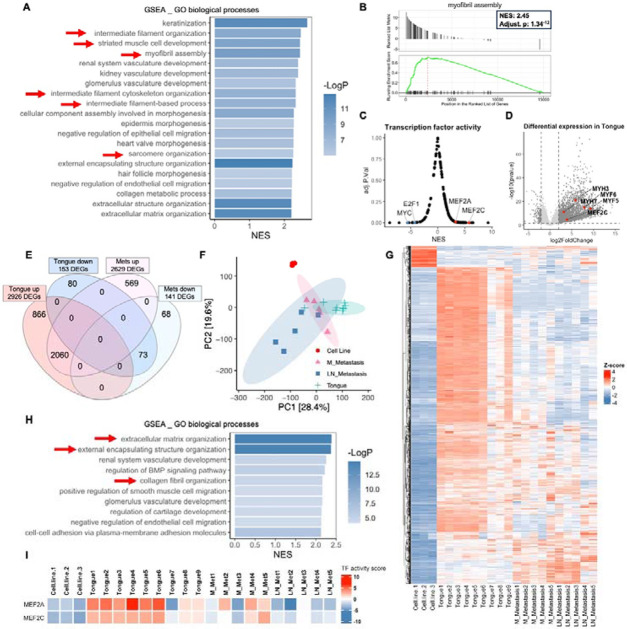
Figure 5. Shifts in differentiation transcriptional signatures between primary and metastatic tumors in the tongue orthotopic xenograft model both compared to cells in culture.
(A) GSEA analysis of bulk RNA-seq shows that RD tongue orthotopic xenografts are enriched for myogenic differentiation/development and myofibril assembly signatures in comparison to RD-EGFP cells in culture (red arrows). Bar chart representation of the top 20 enriched pathways/biological processes ranked by normalized enrichment score (NES). (B) Gene set enrichment (GSEA) plot showing positive, significant enrichment for genes implicated myofibril assembly which are significantly upregulated in RD-EGFP tongue xenograft tumors in comparison to RD-EGFP cells in culture. (C) Transcription factor (TF) activity analysis performed using Dorothea R package shows a significant positive enrichment for downstream targets of the myogenic differentiation transcription factors MEF2C and MEF2A. (D) Volcano plot representation of differentially expression genes (DEGs) in the tongue tumors in comparison to RD-EGFP cells in culture showing examples of DEGs implicated in myogenic differentiation. (E) Venn diagram and (F) PCA plot and (G) heatmap representation of DEGs from tongue tumors show a shift the transcriptional signature between primary tongue tumors and metastatic tumors from cervical lymph nodes and mandible. Venn diagram represents DEGs in the tongue tumors and submandibular metastases (either from the mandible or cervical lymph nodes) using to cells in culture as a reference for both. (H) GSEA analysis of the differential transcriptome of submandibular metastases (from the mandible or cervical lymph nodes) shows the loss of the myogenic development/differentiation signatures and the enrichment for extracellular matrix and collagen organization signatures (red arrows) in metastatic tumors in comparison to tongue tumors (A); transcriptome of cells in culture was used as a reference. (I) TF activity analysis performed using Dorothea R package shows a substantial reduction of MEF2A and MEF2C TF activity in mandibular (M) metastases and to a greater extent in cervical lymph node (LN) metastasis.