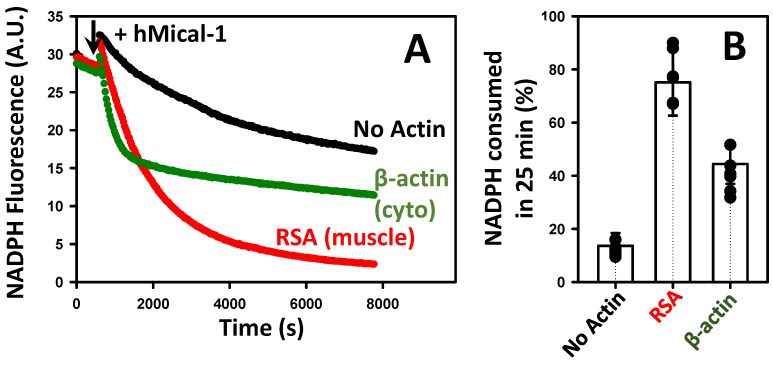
Figure 4.
Cytoplasmic and muscle actins activate Mical-mediated NADPH consumption with different efficiencies. The time-dependent decrease in the fluorescence signal of NADPH is due to its conversion into its oxidized (non-fluorescent) form (NADP+) by Mical enzymes, which is accelerated by F-actin. The traces are color-coded as follows: hMical/NADPH only (no actin) (black triangles), RSA (red circles), and recombinant β-actin (green triangles). Note that different NADPH consumption patterns were observed in the presence of cytoplasmic vs. muscle F-actin. The addition of hMical-1 is indicated by an arrow. Excitation and emission wavelengths were set at 340 nm and 460 nm, respectively. Conditions: [Actin] = 1.5 μM, NADPH = 137 μM, and hMical-1 = 50 nM; N = 4 separate experiments. A representative data set is shown. Each trace corresponds to the average of two technical replicates (two samples) analyzed in the representative experiment shown in the figure. Two different preparations of the recombinant β-actin were tested with similar results. (B) Analysis of the NADPH consumption assays shown in (A). Note that a greater extent of NADPH consumption was observed with muscle (compared to the cytoplasmic) actin at the 25 min time point. Error bars correspond to the standard deviation.