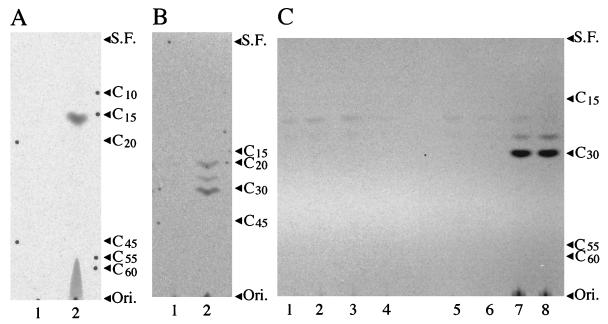
FIG. 1.
Autoradiograms of TLC of the prenyl alcohols obtained by enzymatic hydrolysis (3) of the products formed by the incubations of [1-14C]isopentenyl diphosphate and dimethylallyl diphosphate or FPP with the cell-free homogenates of E. coli transformants. (A) Incubations with dimethylallyl diphosphate. Products were derived from E. coli JM 109/pUC119 (control) (lane 1) and E. coli JM 109/pFP00 (lane 2). (B) Incubations with FPP. Products were derived from E. coli JM 109/pUC119 (control) (lane 1) and E. coli JM 109/pHX00 (lane 2). (C) Incubations with FPP. Products were derived from E. coli JM 109/pUC119 (control) (lane 1), E. coli JM 109/pREG1 (lane 2), E. coli JM 109/pREG2 (lane 3), E. coli JM 109/pREG3S (lane 4), the mixture of E. coli JM 109/pREG1 and E. coli JM 109/pREG2 (lane 5), the mixture of E. coli JM 109/pREG2 and E. coli JM 109/pREG3S (lane 6), the mixture of E. coli JM 109/pREG1 and E. coli JM 109/pREG3S (lane 7), and E. coli JM 109/pHX06 (lane 8). Each extract was analyzed by reversed phase TLC (type LKC-18; Whatman) with solvent systems of acetone-water (9:1 [B] or 19:1 [A and C]). Arrowheads indicate the positions of authentic prenyl alcohols as follows: C10, geraniol; C15, (all-E)-farnesol; C20, (all-E)-geranylgeraniol; C30, (all-E)-hexaprenol; C45, (all-E)-nonaprenol (solanesol); C55, beturaprenol-55, C60, beturaprenol-60; Ori., origin; S.F., solvent front.