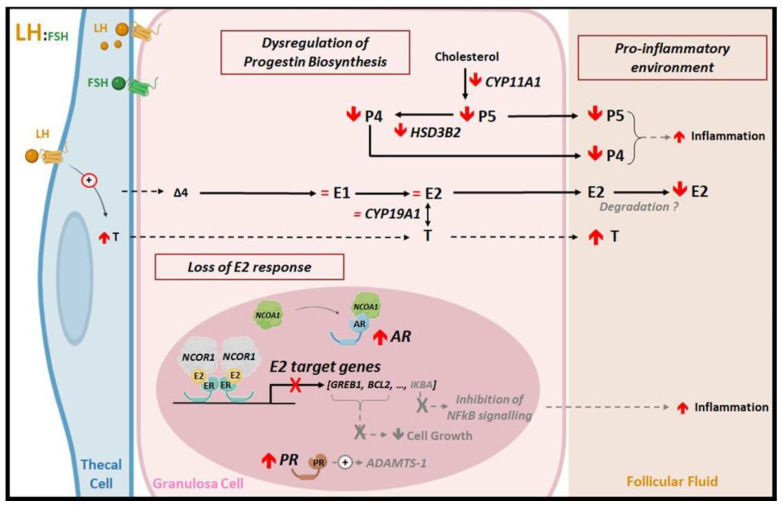
Figure 5.
Proposed model of PCOS ovarian context. Our findings demonstrate the deregulation of progestin biosynthesis in the GCs of PCOS that may be responsible for the lower concentrations of pregnenolone (P5) and progesterone (P4) in the follicular fluid (FF). By contrast, the lower level of estradiol (E2) in the FF of PCOS is not associated with a decline in E2 biosynthesis in GCs, but more probably with E2 degradation in the FF. In addition, our study reveals that E2 lost the ability to regulate the expression of E2 target genes in PCOS, such as GREB1 (growth regulating estrogen receptor binding 1), BCL2 (B-cell lymphoma-2) or IKBA (NF-kappa-B inhibitor alpha). Among the various hypotheses, increased expression of NCOR1 (nuclear receptor corepressor-1) in PCOS might be implicated in the inhibition of estrogen receptor (ERs) transactivities. In that context, E2 could become unable to control GCs growth and contribute to the inflammatory environment of antral PCOS follicles. ADAMTS-1, a disintegrin-like metalloprotease with thrombospondin type motifs-1; AR, androgen receptor; CYP11A1, cholesterol chain cleavage enzyme; CYP19A1, aromatase; ∆4, delta-4 androstenedione; E1, estrone; ER, estrogen receptor; FSH, follicle-stimulating hormone; HSD3B2, 3β-hydroxysteroid dehydrogenase; LH, luteinizing hormone; NCOA1, or SRC-1 for steroid receptor coactivator-1; NFκB, nuclear factor-kappa B; PR, progesterone receptor; T, testosterone. Black full arrows show new deregulations demonstrated in the study along with known processes; black dashed arrows and grey writing show proposal hypothesis.