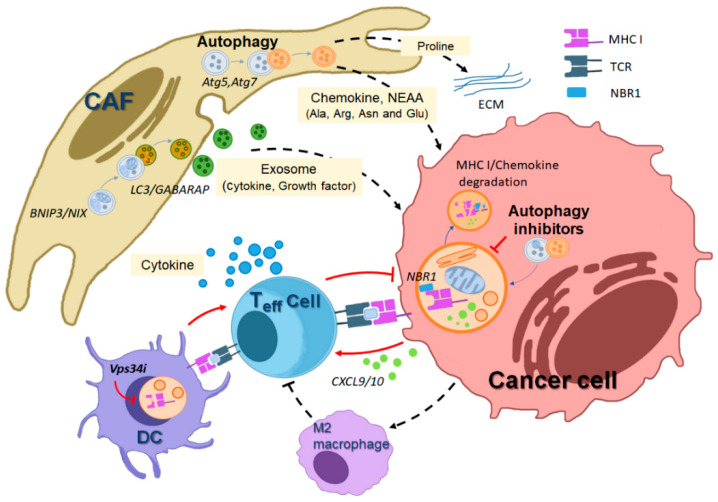
Figure 3.
Illustration depicting how autophagy in various host cells within the tumor microenvironment (TME) mediates crosstalk that supports tumor promotion. Autophagy inhibition affects the anti-tumorigenic roles of diverse host cells in the TME. In host stromal cells, such as cancer-associated fibroblasts (CAFs), autophagy promotes the generation of cellular metabolites, including amino acids derived from macromolecule degradation, supporting tumor cell growth and survival. Additionally, CAFs generate exosomes in an LC3/GABARAP-mediated manner, which are secreted and contain pro-tumorigenic growth factors and cytokines, further promoting tumor cell proliferation. Autophagy-driven pro-collagen degradation and proline production also contribute to extracellular matrix (ECM) production, facilitating tumor proliferation. In host immune cells adjacent to tumors, autophagy plays a role in the degradation of MHC class I mediated by NBR1, an autophagy receptor, thereby inhibiting cytotoxic T cell-mediated tumor killing. Autophagy inhibition in dendritic cells (DCs) or cancer cells leads to increased MHC-I levels on the cell surface by preventing autophagic degradation, thus supporting cytotoxic T cell-mediated cancer death. The black dotted line indicates a tumor-promoting effect, while the red line represents a tumor-suppressing effect. (MHC I; major histocompatibility complex I, TCR; T cell receptor, NBR1; neighbor of the Brca1 gene).