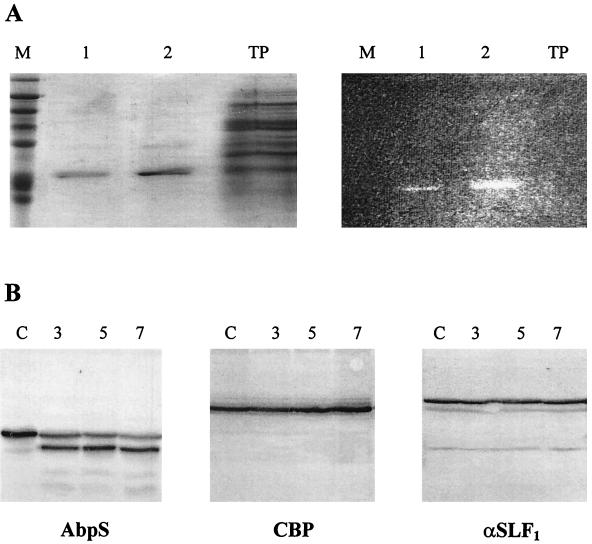
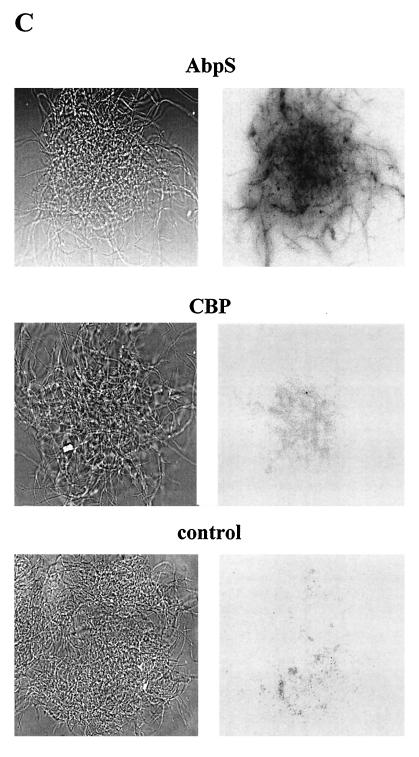
FIG. 5.
Localization of AbpS. (A) S. reticuli mycelia were incubated with FITC, and AbpS was subsequently isolated by its interaction with Avicel and separated by SDS-PAGE. Lanes M, molecular mass standards; AbpS isolated from 0.1 mg of total protein; lanes 2, AbpS isolated from 1 mg of total protein; lanes TP, 10 μg of total protein. The gel was analyzed under UV light (right gel) or after Coomassie brilliant blue staining (left gel). (B) Mycelia from S. reticuli grown with glucose as the sole carbon source were washed and incubated for 3 (lanes 3), 5 (lanes 5), and 7 (lanes 7) h in a proteinase K-containing buffer and for 7 h without proteinase K (lanes C). After being washed, crude cell extracts were prepared. Aliquots (10 μg) of each sample were subjected to SDS-PAGE. After being blotted, the proteins were incubated with antibodies raised against AbpS (left blot), CBP (middle blot) (28), or α-SLF1 (right blot) (8). (C) Washed S. reticuli mycelia were incubated with anti-AbpS antibodies (top section) and anti-CBP antibodies (middle section), or without primary antibodies (bottom section) for 4 h at room temperature. After unbound antibodies were removed, secondary FITC-labelled anti-rabbit IgG2ab was used to determine the presence of bound primary antibodies on the surfaces of S. reticuli hyphae, with the help of UV light and an Axiovert microscope.