Abstract
To acquire the capacity to fertilize the oocyte, mammalian spermatozoa must undergo a series of biochemical reactions in the female reproductive tract, which are collectively called capacitation. The capacitated spermatozoa subsequently interact with the oocyte zona-pellucida and undergo the acrosome reaction, which enables the penetration of the oocyte and subsequent fertilization. However, the spontaneous acrosome reaction (sAR) can occur prematurely in the sperm before reaching the oocyte cumulus oophorus, thereby jeopardizing fertilization. One of the main processes in capacitation involves actin polymerization, and the resulting F-actin is subsequently dispersed prior to the acrosome reaction. Several biochemical reactions that occur during sperm capacitation, including actin polymerization, protect sperm from sAR. In the present review, we describe the protective mechanisms that regulate sperm capacitation and prevent sAR.
Keywords: spermatozoa, capacitation, acrosome reaction, spontaneous acrosome reaction, actin polymerization, signaling
1. Introduction
Prior to penetrating the oocyte, mammalian spermatozoa should undergo a highly regulated process called the acrosome reaction (AR). The physiological AR is a precise regulated Ca2+-dependent exocytotic process induced by the sperm–oocyte contact, causing a rapid increase in intracellular Ca2+ concentrations, thereby initiating the AR [1,2]. It is generally accepted that the physiological AR occurs as a result of the interaction of intact sperm with the oocyte zona-pellucida (ZP). Florman and Storey suggested that the ZP is the site of the AR in mice [3], though it was also suggested that mouse sperm begin to undergo the AR in the upper isthmus of the oviduct [4]. During IVF in mice, acrosome-intact sperm remain attached to the ZP for a longer time than reacted sperm, thereby facilitating fertilization [5,6]. However, it has been suggested that mouse sperm that undergo the AR before contact with the oocyte ZP can still fertilize the oocyte [7]. Pre-treatment of bovine sperm with ZP-glycoproteins causes an increase in the AR and significantly inhibits the subsequent penetration of these sperm into the oocyte, suggesting that the AR occurs after the initial interaction between the sperm and the oocyte, at least in cows [8]. ZP isolated from various species are able to induce the AR in mice, hamsters, guinea pigs, rabbits, cows, monkeys and humans [9].
Before initial contact with the oocyte and in order to undergo the AR, mammalian sperm must first undergo several biochemical processes in the female reproductive tract, which are collectively called capacitation (rev. in [10]). Our group demonstrated that actin polymerization occurs during sperm capacitation and that the F-actin is then dispersed prior to the AR [11]. Inhibition of F-actin formation during sperm capacitation results in the spontaneous acrosome reaction (sAR) [12]. The sAR is a premature form of the AR that does not lead to productive fertilization. It is defined as an AR that occurs in sperm incubated under capacitation conditions but without any AR-inducer, while the physiological AR is defined as an AR that occurs in capacitated sperm after induction by ZP or by other known inducers such as Ca2+-ionophores or progesterone. Morphologically, the sAR appears similar to the induced AR. However, sperm samples with a high proportion of cells that have undergone the sAR result in poor success in human IVF [13]. In varicocele patients, the autoimmune antisperm reaction is accompanied by the presence of the sAR and a lack of induced reactions and an increase in intracellular reactive oxygen species (ROS) concentration and DNA fragmentation [14]. Sperm in obese men show a low fertility rate and elevated sAR levels, which are associated with altered circulating levels of estradiol (E2) and sperm cholesterol content [15]. A similar increase in the sAR was seen in spermatozoa from mice fed a high-fat diet [16]. These results suggest that a decrease in E2 and fatty acid levels may influence spermatogenesis [17] and may affect some steps of acrosome biogenesis that will have consequences for fertilization. The molecule 2-arachidonoylglycerol (2AG) affects the in vitro functionality of human sperm by reducing motility, inhibiting capacitation and triggering the sAR [18]. It was shown in human sperm that 2AG inhibits the Ca2+-channel CatSper and accumulates in the cell when the progesterone-dependent lipid hydrolase ABHD2 is blocked [19].
The degree of the sAR in human sperm may have clinical importance in predicting the results of IVF, as it is negatively correlated with the achievement via IVF of high-quality embryos and pregnancy rate [20]. Loading sterols into chicken spermatozoa before cryopreservation enhances their quality by inhibiting early apoptotic changes and the sAR [21]. However, sperm from polyzoospermic men demonstrate a low sAR rate as well as low levels of Ca2+-ionophore (A23187)-induced AR [22,23,24]. Nevertheless, in boar sperm, the percentage of the sAR was not significantly different in fertile (4.5%) versus subfertile boars (4.75%) [25]. Thus, there are differences among various species regarding the correlation between the sAR and fertilization rate.
In mice, the sAR renders spermatozoa fertilization incompetent [3]. Moreover, an intact acrosome is required for the chemotaxis of mouse spermatozoa towards the oocyte [26], indicating that spermatozoa that undergo the sAR before reaching the oocyte cumulus oophorus are unlikely to respond to the oocyte chemotactic signals. Suarez showed that 98% of rabbit sperm collected from the oviduct ampulla at the beginning of fertilization were acrosome-intact [27], suggesting that acrosome-reacted sperm are unable to penetrate the oviduct. Thus, to achieve fertilization, the sperm must prevent the AR from occurring before contact with the oocyte. The goal of this review is to describe the known mechanisms that protect spermatozoa from the sAR.
2. Role of Actin Polymerization
The conversion of G-actin to F-actin is a necessary process to achieve sperm capacitation [11,28]. This process of actin polymerization is mediated by phospholipase D (PLD) and the kinases protein kinase A (PKA) and tyrosine kinase [28], two key kinases involved in the capacitation process [29,30] (see Figure 1). At least three tyrosine kinases are involved in sperm capacitation: Src, Pyk2 and Fer (see Figure 1). We showed in bovine sperm that the PKA and protein kinase C (PKC)-dependent signal transduction pathways can potentially lead to PLD activation; however, under physiological capacitating conditions, actin polymerization depends primarily on PKA activation [28]. The activation of PKA during capacitation causes the inactivation of phospholipase C (PLC), preventing PKC activation [28] (see Figure 1). The role of PLC is to hydrolyze phosphatidyl-inositol-4,5-bisphosphate (PIP2) to diacylglycerol, which activates PKC, and inositol-triphosphate (IP3), which in turn activates the Ca2+ channel in the outer acrosomal membrane, causing Ca2+ release from the acrosome, further activating the Ca2+-activated PKC.
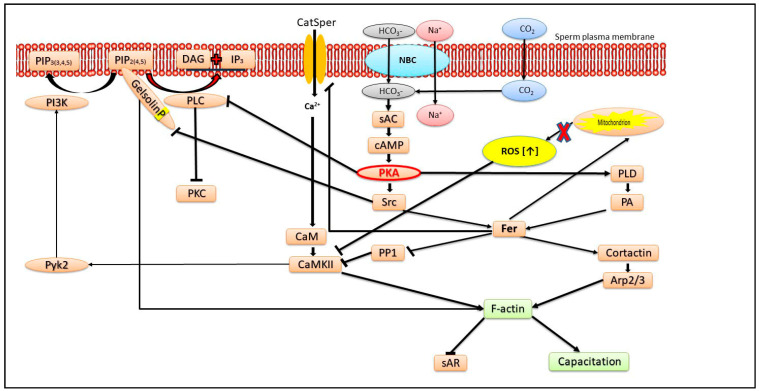
Figure 1.
The mechanisms that protect sperm from sAR: All pathways that lead to F-actin formation and complete capacitation protect sperm from sAR. The main factor is protein-kinase A (PKA) activated by cAMP generated by HCO3-activated soluble adenylyl-cyclase (sAC). The initial entrance of HCO3− is mediated by Na+/H+ cotransporter belonging to the SLC4 family or by the conversion of CO2 to HCO3− by carbonic-anhydrase. PKA inhibits phospholipase C (PLC), preventing phosphatidyl-inositol-4,5-bisphosphate (PIP2) hydrolysis and promoting the interaction between gelsolin and PIP2, leading to gelsolin tyrosine phosphorylation by Src and maintaining its inactive state allowing F-actin formation. The activation of Src and phospholipase D1 (PLD) by PKA also causes Fer activation, which promotes F-actin formation by two pathways: Fer activates cortactin, leading to Arp2/3 activation, and also inhibits the protein phosphatase 1 (PP1), causing calmodulin-kinase II (CaMKII) activation, resulting in F-actin formation. Fer also prevent production of too much ROS by the mitochondria and regulates Ca2+ transport via CatSper, leading to CaMKII activation and preventing relatively high Ca2+ influx, which causes sAR. Active CaMKII activates the tyrosine kinase Pyk2, which phosphorylates phosphtidyl-inositol-3-phosphate (PI3K), promoting PIP2 formation and increasing the opportunity of gelsolin to interact with PIP2, which causes gelsolin inhibition as described above. In the flagellum, Fer also regulates complex I of the mitochondrial electron transport chain, thereby restraining ROS production and preventing CaMKII inhibition.
PKA activation promotes F-actin formation and sperm capacitation, whereas the premature activation of PKC during capacitation jeopardizes this process. Indeed, PKA mediates PLD activation and the inhibition of PKA, resulting in an increase in the sAR and a decrease in F-actin levels, and these two activities can be reversed by adding phosphatidic acid vesicles, the product of PLD activity [31]. The activation of PKC in mouse sperm showed no effect on the sAR [32]. However, the addition of bicarbonate to equine or hamster sperm, which activates the soluble adenylyl cyclase to produce cAMP, leading to PKA activation, causes an increase in the sAR [33,34] in contrast to the findings in bovine sperm described above. Hyperactivated (HA) human spermatozoa generally show high levels of capacitation and display greater sAR levels than non-HA spermatozoa during incubation in synthetic culture media [35]. Moreover, aryl hydrocarbon receptor-KO spermatozoa were frequently capacitated and showed reduced sAR levels and very limited actin polymerization during capacitation [36]. These results in mouse sperm contradict the data in bovine sperm, in which the inhibition of PKA inhibits actin polymerization and capacitation resulted in an increase in the sAR [31]. It is therefore possible that low F-actin levels prevent capacitation in bovine but not in mouse sperm.
In human sperm, the hydrolysis of PIP2 by PLC prior to the AR causes the release of the bound actin-severing protein gelsolin, which disperses F-actin, allowing the AR to occur [37]. During capacitation, gelsolin is bound to PIP2 and undergoes tyrosine phosphorylation by sarcoma-protein kinase (Src), two processes which keep gelsolin inactive during capacitation, allowing the formation of F-actin [37] (see Figure 1), which protects the sperm from the sAR.
Phosphatidyl-inositol-3-kinase (PI3K) is phosphorylated on tyrosine-467 during bovine sperm capacitation and mediates F-actin formation only when PKA is highly activated (by the addition of exogenous cAMP to the sperm) [38]. PKA activates the tyrosine kinase Src, which inhibits protein-phosphatase 1 (PP1), leading to Ca2+/calmodulin-dependent protein kinase II (CaMKII) activation. This in turn mediates PI3K-tyrosine phosphorylation by activating the tyrosine-kinase Pyk2 [39] (see Figure 1). We showed that PKA phosphorylates glycogen-synthase kinase-3 (GSK3), causing its inactivation, leading to low sAR levels. This suggests that the maintenance of acrosome reaction timing is mediated by PKA via the regulation of GSK-3 beta activity [40]. In a recent study, we also showed in human sperm that the inhibition of PP1 by Src is mediated by the tyrosine kinase FER, which is activated by PKA/Src activities [41]. Src-family-kinase (SFK) phosphorylation in bird sperm inhibits the sAR, but interestingly, in birds sperm, SFK is not localized downstream to PKA and is primarily regulated by calcium-dependent tyrosine phosphatase activity [42].
CaMKII induces F-actin formation either by inducing actin polymerization or by stabilizing actin fibers [43]. It was shown that active CaMKII prevents the sAR in mouse sperm by interacting with the multi-PDZ-domain protein1, MUPP1 [44]. The inhibition of CaMKII in bovine sperm results in the sAR, and this effect is reversed by the activation of PLD by spermine [12]. Spermine activates phosphatidyl-inositol-4-kinase (PI4K), resulting in an increase the level of PIP2, which is a cofactor for PLD activation. Furthermore, the decrease in F-actin and the increase in the sAR by the inactivation of the PLD pathway can be reversed by CaMKII activation using H2O2 or PP1 inhibition [12], and the recovery by PP1 inhibition is mediated by PI3K [31]. In order to fully activate actin polymerization and prevent the sAR, both forms of CaMKII, p-CaMKII and oxidized CaMKII should be activated [12]. These results indicate that two distinct pathways, involving PLD or CaMKII, lead to F-actin formation during capacitation and protect the sperm from the sAR.
We found that Ezrin activity during sperm capacitation mediates actin polymerization and prevents the occurrence of the sAR in bovine sperm [45]. Ezrin, Radixine and Merlin are closely related proteins called ERM proteins, which form cross links between the plasma membrane and actin and thereby mediate actin polymerization in cells. Ezrin is highly phosphorylated/activated during the first hour of the capacitation process, and subsequently, its phosphorylation rate is significantly decreased. Ezrin phosphorylation depends on protein PKA and CaMKII activities and to some extent on PI4K activity. The inhibition of these three kinases stimulates the sAR, in which the effect of PI4K inhibition, but not the inhibition of PKA or CaMKII, can be reversed by increasing p-Ezrin using a phosphatase inhibitor [45].
3. Ca2+ Transport Mechanisms Regulates the AR
Extracellular Ca2+ is required to trigger the sAR in human spermatozoa [46]. A relatively low extracellular Ca2+ concentration (~30 µM) is required for sperm capacitation’ however, Ca2+ is not required for protein tyrosine phosphorylation, and high [Ca2+]i (0.15 mM) decreases the protein tyrosine phosphorylation levels [47]. Relatively high [Ca2+]i induces the sAR, and therefore the sperm must precisely regulate its [Ca2+]i. Calcium channels, including the sperm-specific cation channel CatSper, induce the sAR in human and bovine sperm [41,48]. The inhibition of the Ca2+-ATPase of the outer acrosomal membrane using thapsigargin results in Ca2+ release from the acrosome to the cytosol via IP3R, leading to Ca2+ influx into the cell via the Ca2+-dependent Ca2+ channel (CDCC) of the plasma membrane [49], which triggers the sAR [50]. Thus, the Ca2+-ATPase of the outer-acrosomal membrane protects sperm cells from the sAR by maintaining a relatively high [Ca2+] inside the acrosome and preventing CDCC activity.
NMDA-type glutamate receptor mediates the sAR in newt sperm by increasing Ca2+ transport into the cells [51]. Furthermore, in human sperm, the inhibition of the sperm-specific potassium channel, KSPER, decreased the level of sAR [52]. In mouse sperm, KSPER and CatSper together account for all cation currents activated by voltage and alkalization [53] and are thought to act in concert to mediate the changes in membrane cation conductance and Ca2+ influx that occur during the onset of capacitation [54].
The decapacitation mechanism of the seminal-vesicle-auto-antigen might target membrane sphingomyelin and regulate the plasma membrane Ca2+-ATPase activity to reduce the intracellular Ca2+ concentration, thereby reducing the cAMP level and preventing the sAR [55].
Other mechanisms involving Ca2+ may also inhibit the sAR. It was suggested that pH-dependent Ca2+ oscillations prevent premature sAR in human sperm [56,57]. In addition, it was shown that ~30% of human sperm display spontaneous Ca2+ oscillations correlated with the absence of the AR, suggesting another mechanism reducing the occurrence of the sAR. It was also suggested that protein–protein interactions between the Ca2+-sensor protein synaptotagmin [58] and SNARE-associated complexin [59,60,61] maintain the membrane fusion machinery at an intermediate pre-fusion stage [62], thereby preventing the sAR [63].
In analogy to Ca2+ transport, H+ transport mechanisms may also affect the sAR. Alkalization of the intra-acrosomal space was shown to cause an increase in the sAR [64]. These authors suggested that the vacuolar-type H1 ATPase (V-ATPase), the Na+/H+ exchanger (NHE) and the Cl−/HCO3− exchanger maintain the acidic pH in the acrosome and prevent inner-acrosomal alkalization and the sAR [64]. Conversely, cytosol alkalization leads to CatSper activation [65] and to elevated sAR levels (our unpublished data). In conclusion, Ca2+ transport mechanisms mediate the sAR.
4. Role of Reactive Oxygen Species (ROS) and Mitochondrial Activity in the sAR
Oxidative stress is currently considered to be a main cause of male infertility (rev. by [66]). Although presence of a basal level of ROS is essential for the onset of sperm-activating processes such as capacitation [67], its increased levels disturb sperm functions, thereby leading to male infertility by mechanisms such as lipid peroxidation and DNA damage [68]. The levels of ROS are therefore precisely regulated in sperm, mainly by superoxide dismutase (SOD), which coverts superoxide anions to H2O2 [69], and by catalase [70], which decomposes H2O2. Reactive oxygen species (ROS) are formed during sperm capacitation, which is important for the activation of CaMKII [12] and PLD [31]. Our group showed that treatment of bovine sperm with 50 µM H2O2 causes a significant increase in CaMKII phosphorylation/activation, a state that is completely reversed by 100 µM H2O2 [71]. In human sperm, the addition of SOD causes a decrease in the sAR [72]. In bovine sperm, hydrogen peroxide promotes capacitation, mimicking the role of bicarbonate in activating the soluble adenylate cyclase to activate the cAMP/PKA [73]. Also, ROS have been implicated in protein tyrosine phosphorylation, which mediates capacitation in several species [74]. Nevertheless, in boar sperm, ROS do not promote capacitation but stimulate the sAR [75].
In our recent study, we showed in human sperm that the activated tyrosine kinase FER enhances actin polymerization and protects sperm from the sAR [41]. Activated FER acts on several levels; it inhibits PP1 and regulates Ca2+ influx via CatSper, leading to CaMKII activation and actin polymerization. Simultaneously, FER also activates cortactin, leading to Arp2/3 activation and F-actin formation. In addition, FER regulates mitochondrial respiration via complex I of the electron transport chain [76] and restrains ROS production, thereby preventing CaMKII inhibition by high levels of ROS.
The knockout of several genes, including β-Defensin, the Lipocalin family LCN8 [51] or Aldehyde-dehydrogenase ALDH4A1, a key enzyme in mitochondrial prolin metabolism [77], results in an increase in mouse sperm sAR levels. However, the upregulation of cytochrome C in pig sperm promotes the sAR, indicating that mitochondrial activity stimulates the sAR [78]. This observation supports our notion regarding the regulation of the mitochondrial electron transport chain complex I by FER, whereby its inhibition promotes the sAR [41]. The regulation of the mitochondrial electron transport chain controls the production of ROS and protects the sperm from the sAR. Thus, FER, as an important regulator of mitochondrial activity is responsible for providing ATP for various sperm functions, leading to proper fertilization [41].
Interestingly, to prevent spermatozoa from potential oxidative stress damage, and probably from the sAR, the fatty-acid composition of rodent sperm membranes is altered by increasing the percentage of peroxidation-resistant fatty acids under competitive conditions [79].
Paraoxonase 1 (PON1) is a high-density lipoprotein-associated enzyme that acts as an antioxidant [80]. We showed that PON1 protects human sperm from the sAR [81]. Endogenous semen PON1 activity is negatively associated with the sAR, suggesting that PON1 protects against the sAR by reducing ROS levels [81]. It was also shown that a reduction in PON1 levels in semen is associated with infertility [82].
5. Role of Energy Metabolism in the sAR
It is well known that sperm ATP is produced by glycolysis and mitochondrial respiration. The inhibition of either glycolysis or oxidative phosphorylation in bovine sperm does not affect capacitation or sAR levels; however, when both systems are inhibited, no capacitation occurs, and there is a significant increase in sAR levels [48]. Under such ATP starvation, the increase in the sAR is triggered by Ca2+ influx into the sperm via the CatSper cation channel. There is no change in PKA activity when glycolysis or mitochondrial respiration is inhibited, while a complete reduction in PKA activity was observed when both systems were inhibited [48]. Protein tyrosine phosphorylation (PTP), also known to increase during sperm capacitation, was partially reduced by the inhibition of one metabolic system and completely blocked when the two metabolic systems were inhibited [48]. These studies show that ATP, PKA and PTP are involved in the mechanisms protecting sperm from the sAR.
In pig sperm, the levels of the β-subunit of H+-ATPase, the ATP-producing enzyme in the mitochondria, isocitrate-dehydrogenase (IDH) and pyruvate-dehydrogenase are enhanced during capacitation, while the level of enolase, a critical enzyme in anaerobic glycolysis, is decreased [78]. IDH is the main regulatory enzyme of the Krebs cycle, and its increase during capacitation indicates the involvement of the malate–aspartate shuttle required to maintain the levels of reduced NADP necessary for capacitation. Thus, mitochondrial and glycolytic activities are involved in the mechanism of sperm capacitation and protect sperm from the sAR.
6. Role of Zn2+ in the sAR
Zinc ions play an important role in the male reproductive system [83,84,85]. Zn2+ has antibacterial activity and can kill both Gram-positive and Gram-negative bacteria [86,87]. It was shown that Zn2+-deficient nutrition causes male infertility [88]. Zinc ions are secreted to the semen mainly from the prostate [89]. The addition of Zn2+ to semen extender before freezing sperm reduces ROS levels and increases the yield of fertilization after sperm thawing [90], but excess Zn2+ can increase ROS levels, resulting in the sAR [91]. It has been shown that capacitation-induced Zn2+ efflux allows sperm release from oviductal glycan by activating Zn-containing enzymes, such as metalloproteinase2, involved in sperm penetration of the ZP [92].
Extracellular Zn2+ interacts with the sperm Zn2+-sensing receptor (ZnR) also named GPR39 [93], which is localized in the sperm tail and the acrosome [94,95,96], suggesting its possible involvement in motility and the AR. We showed that Zn2+ mediates the GPR39-dependent bovine sperm AR [96] and human sperm hyper-activated motility/capacitation [94] (see Figure 2). Thus, the positive effect of Zn2+ on sperm capacitation protects sperm from the sAR [12].
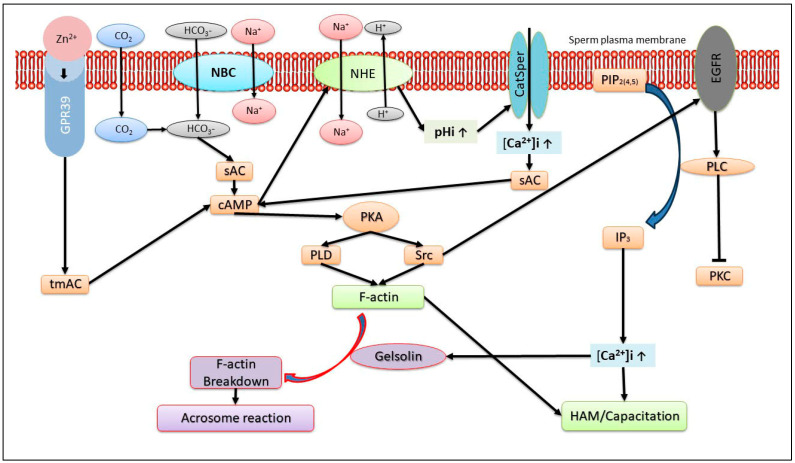
Figure 2.
The mechanism of action of Zn2+ in sperm capacitation and the acrosome reaction. The binding of Zn2+ to the sperm receptor GPR39 activates trans membrane adenylyl-cyclase (tmAC) to produce cAMP, which activates the Na+/H+ exchanger (NHE), resulting in intracellular alkalization and CatSper activation. The elevation of intracellular Ca2+ concentration together with HCO3− activates the soluble adenylyl-cyclase (sAC), which further enhances the level of cAMP, leading to protein-kinase A (PKA) activation. PKA activates the tyrosine-kinase Src, followed by epidermal growth factor receptor (EGFR) activation, and that of its downstream effector, phospholipase Cγ (PLC), which catalyzes the hydrolysis of phosphatidyl-inositol-4,5-bisphosphate (PIP2) to produce inositol-tri-phosphate (IP3) and diacylglycerol. IP3 activates the IP3 receptor of the outer acrosomal membrane to release Ca2+ from the acrosome to the cytosol, promoting the development of hyper-activated motility and capacitation. PKA also activates phospholipase D1 (PLD), which promotes F-actin formation and capacitation. At the end of capacitation and prior to the acrosome reaction, there is an increase in intracellular Ca2+ concentration to the µM range, which further activates PLC to hydrolyze PIP2, inducing the release of PIP2-bound gelsolin, which is activated by Ca2+, and breakdown of F-actin, enabling the acrosome reaction.
GPR39 belongs to the G-protein-coupled receptor (GPCR) family, which activates trans-membrane adenylyl cyclase (tmAC), and the resulting cAMP activates PKA, a key enzyme in sperm capacitation. We demonstrated the involvement of two GPCRs, angiotensin-II-receptor and lysophosphatidic-acid receptor, in bovine sperm capacitation [97]. It has been suggested that Zn2+ can stimulate the activity of tmACas well as sAC [94] (see Figure 2). A relatively low concentration of Zn2+ (5 µM) causes an increase of about 40% in intracellular cAMP levels [96], but higher concentrations (20–30 µM) have a lesser effect [94]. These effects of different Zn2+ concentrations on intracellular cAMP levels correlate closely with the relatively higher stimulation of hyper-activated/capacitation at low concentrations of Zn2+ [94]. As described above, actin polymerization during sperm capacitation is essential for preventing the sAR. We found that the addition of 5 µM Zn2+ to bovine sperm increases the actin polymerization rate and decreases the sAR (unpublished). These results further support the significant effect of Zn2+ in protecting sperm from the sAR.
In Figure 2, we present a model summarizing the mechanisms that regulate human and bovine sperm capacitation mediated by Zn2+. The increase in intracellular cAMP by Zn2+ stimulates the Na+/H+ exchanger [98], which increases intracellular PH, leading to CatSper activation. Thus, the zinc ion stimulates hyper-activated motility/capacitation through the CatSper-dependent activation of the AC→cAMP→PKA→Src→EGFR and PLC cascade [94]. In bovine sperm capacitation, Zn2+ stimulates EGFR, which is mediated by the activation of tmAC→cAMP→PKA→Src [96]. In capacitated sperm, Zn2+ further stimulates the EGFR and the downstream effectors PI3K, PLC and PKC, resulting in the acrosome reaction [96] (see Figure 2). The hydrolysis of PIP2 by PLC generates IP3, which activates IP3R localized in the outer acrosomal membrane and in the redundant nuclear envelope (RNE), resulting in Ca2+ release from the acrosome and RNE, promoting the development of hyper-activated motility/capacitation [99]. This cascade can be initiated by Zn2+-activated GPR39, leading to PLC activation.
7. Role of Protein Acetylation in the sAR
In a recent study, we showed that protein hyperacetylation protects bovine sperm from the sAR through an exchange protein directly activated by cAMP (EPAC) and via CaMKII-dependent and PKA-independent mechanisms [100]. Protein acetylation, including tubulin acetylation, is involved in sperm energy metabolism and motility [101]. Recently, several studies have described changes in the levels of acetylated proteins during human sperm capacitation [102,103]. Different protein acetylation profiles were observed in sperm during capacitation versus fertilization, suggesting that protein acetylation is involved in the fertilization process [103]. Changes in protein acetylation are also seen during axonemal microtubule construction [104,105], suggesting that poor sperm motility and male infertility may be associated with perturbed tubulin acetylation [105]. Moreover, hyperacetylation in non-capacitated mouse sperm induces capacitation-associated molecular events, including the activation of PKA and of the sperm-specific Ca2+-channel CatSper, hyperpolarization of the plasma membrane, hyperactivated motility and an increase in the AR [106]. Incubation of bovine sperm under non-capacitated conditions revealed a significant increase in the sAR that was reduced in the presence of deacetylase inhibitors, which caused protein hyperacetylation [100].
It was shown that EPAC mediates human and mouse AR [107,108]. We showed that the inhibition of PKA induces an EPAC-dependent AR [50]. Moreover, the induction of the AR by progesterone, angiotensin II, thapsigargin or Ca2+-ionophore is also mediated by EPAC, suggesting a physiological role of EPAC in the AR mechanism [100].
8. Additional Factors Regulating sAR
Several other factors were also shown to affect the sAR. These will be covered, in brief, below. Bacterial contamination: Bovine sperm incubated with the bacteria Escherichia coli (E. coli), Staphylococcus aureus (S. aureus) or Pseudomonas aeruginosa (P. aeruginosa) revealed a sperm–bacteria interaction; however, only E. coli and P. aeruginosa caused an increase in sperm sAR levels [109]. In addition, PKA and protein tyrosine phosphorylation activities were inhibited by the bacteria. Moreover, increasing intracellular cAMP, which also occurs during sperm capacitation, caused a significant reduction in the sAR induced by the bacteria [109]. Thus, the increase in the sAR by bacterial contamination in the semen or in the female reproductive tract could provide a possible explanation for infertility. It was further shown that disruption of in vivo β-defensins, an antimicrobial peptide, alters intracellular calcium levels, which leads to the sAR [110,111].
During induced AR and sAR in mouse sperm, IZUMO1 translocates from the acrosomal cap to the equatorial segment and further spreads over the whole sperm head. Moreover, protein tyrosine phosphorylation in the tail occurs at the beginning of the capacitation, and the progress of IZUMO1 relocation positively correlates with the level of acrosome instability, leading to the sAR [112]. In mammalian fertilization, IZUMO1 binds to its oocyte receptor counterpart, Juno, to facilitate recognition and fusion of the gametes.
sAR levels are elevated in CD46−/− mice, indicating that CD46, which is localized in the inner acrosomal membrane, plays a role in sperm protection against the sAR [113,114,115]. Human membrane cofactor protein CD46 is a ubiquitously expressed protein known to protect cells from complement attack. The absence of CD46 protein expression is associated with acrosomal instability in mice expressing novel CD46 transcripts, resulting in a rapid sAR. This provides a strategy to increase the competitive sperm advantage for individuals, leading to faster fertilization in highly promiscuous genus.
In Lcn8−/− male mice, the proportion of immobilized sperm was elevated in the cauda epididymis, and the sperm sAR frequency was increased [116]. Lcn8 is a member of the lipocalin family, in which Lcn8−/− and Lcn9−/− male mice showed normal spermatogenesis and fertility, while a decreased sperm quality was noticed in Lcn8−/− male mice, including increased morphologically abnormal sperm, attenuated sperm motility and premature acrosome reaction of the sperm in cauda epididymis. These results indicated that Lcn8 deficiency causes epididymal sperm maturation defects in mice.
Sperm express epidermal growth factor receptor (EGFR), and the AR can be induced by EGF, indicating that PLCγ is required for the AR [97]. We also showed that α7 nicotinic acetylcholine receptor (α7nAChR) might be a sperm receptor for the interaction with the oocyte, activated by solubilized ZP to induce an EGFR-mediated AR. Isolated ZP or α7 agonists induced the AR in sperm from WT but not in α7-null mouse spermatozoa, and the induced AR was inhibited by α7 or EGFR antagonists. Moreover, the sAR in α7-null sperm was very low, indicating that the regulation of the AChR serves as a protective mechanism against the sAR [117]. This conclusion was further supported in a more recent study showing that AChR antagonists suppress the sAR induced by acetyl-choline or nicotine [118]. In human sperm, nicotine causes an increase in the sAR; thus, the occurrence of high levels of nicotine in the body and, specifically, in seminal fluid might affect fertilization capacity [119].
These findings that occur in the sAR are vital for understanding the fertilization process.
9. Conclusions
Spermatozoa contain several protective mechanisms that limit the sAR. In general, any defect in a mechanism leading to sperm capacitation might promote the sAR, and, accordingly, some of the processes that occur in capacitation are protective. Such mechanisms include PKA, PLD, CaMKII, tyrosine kinase activities and actin polymerization. Further elucidation of these mechanisms should enable us to optimize fertilization both for IVF in humans and for animal breeding.
Author Contributions
The review was written by H.B. and the Figures were done by E.G. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declair no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Florman H.M., Jungnickel M.K., Sutton K.A. Regulating the acrosome reaction. Int. J. Dev. Biol. 2008;52:503–510. doi: 10.1387/ijdb.082696hf. [DOI] [PubMed] [Google Scholar]
- 2.Jungnickel M.K., Sutton K.A., Wang Y., Florman H.M. Phosphoinositide-dependent pathways in mouse sperm are regulated by egg ZP3 and drive the acrosome reaction. Dev. Biol. 2007;304:116–126. doi: 10.1016/j.ydbio.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Florman H.M., Storey B.T. Mouse gamete interactions: The zona pellucida is the site of the acrosome reaction leading to fertilization in vitro. Dev. Biol. 1982;91:121–130. doi: 10.1016/0012-1606(82)90015-X. [DOI] [PubMed] [Google Scholar]
- 4.La Spina F.A., Puga Molina L.C., Romarowski A., Vitale A.M., Falzone T.L., Krapf D., Hirohashi N., Buffone M.G. Mouse sperm begin to undergo acrosomal exocytosis in the upper isthmus of the oviduct. Dev. Biol. 2016;411:172–182. doi: 10.1016/j.ydbio.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakanishi T., Ikawa M., Yamada S., Parvinen M., Baba T., Nishimune Y., Okabe M. Real-time observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Lett. 1999;449:277–283. doi: 10.1016/S0014-5793(99)00433-0. [DOI] [PubMed] [Google Scholar]
- 6.Baibakov B., Gauthier L., Talbot P., Rankin T.L., Dean J. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development. 2007;134:933–943. doi: 10.1242/dev.02752. [DOI] [PubMed] [Google Scholar]
- 7.Jin M., Fujiwara E., Kakiuchi Y., Okabe M., Satouh Y., Baba S.A., Chiba K., Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA. 2011;108:4892–4896. doi: 10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amari S., Yonezawa N., Mitsui S., Katsumata T., Hamano S., Kuwayama M., Hashimoto Y., Suzuki A., Takeda Y., Nakano M. Essential role of the nonreducing terminal alpha-mannosyl residues of the N-linked carbohydrate chain of bovine zona pellucida glycoproteins in sperm-egg binding. Mol. Reprod. Dev. 2001;59:221–226. doi: 10.1002/mrd.1026. [DOI] [PubMed] [Google Scholar]
- 9.Hirohashi N., Yanagimachi R. Sperm acrosome reaction: Its site and role in fertilization. Biol. Reprod. 2018;99:127–133. doi: 10.1093/biolre/ioy045. [DOI] [PubMed] [Google Scholar]
- 10.Visconti P.E., Krapf D., de la Vega-Beltran J.L., Acevedo J.J., Darszon A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J. Androl. 2011;13:395–405. doi: 10.1038/aja.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brener E., Rubinstein S., Cohen G., Shternall K., Rivlin J., Breitbart H. Remodeling of the actin cytoskeleton during mammalian sperm capacitation and acrosome reaction. Biol. Reprod. 2003;68:837–845. doi: 10.1095/biolreprod.102.009233. [DOI] [PubMed] [Google Scholar]
- 12.Shabtay O., Breitbart H. CaMKII prevents spontaneous acrosomal exocytosis in sperm through induction of actin polymerization. Dev. Biol. 2016;415:64–74. doi: 10.1016/j.ydbio.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Fenichel P., Donzeau M., Farahifar D., Basteris B., Ayraud N., Hsi B.L. Dynamics of human sperm acrosome reaction: Relation with in vitro fertilization. Fertil. Steril. 1991;55:994–999. doi: 10.1016/S0015-0282(16)54312-X. [DOI] [PubMed] [Google Scholar]
- 14.Bozhedomov V.A., Lipatova N.A., Rokhlikov I.M., Alexeev R.A., Ushakova I.V., Sukhikh G.T. Male fertility and varicocoele: Role of immune factors. Andrology. 2014;2:51–58. doi: 10.1111/j.2047-2927.2013.00160.x. [DOI] [PubMed] [Google Scholar]
- 15.Samavat J., Natali I., Degl’Innocenti S., Filimberti E., Cantini G., Di Franco A., Danza G., Seghieri G., Lucchese M., Baldi E., et al. Acrosome reaction is impaired in spermatozoa of obese men: A preliminary study. Fertil. Steril. 2014;102:1274–1281.e2. doi: 10.1016/j.fertnstert.2014.07.1248. [DOI] [PubMed] [Google Scholar]
- 16.Bunay J., Gallardo L.M., Torres-Fuentes J.L., Aguirre-Arias M.V., Orellana R., Sepulveda N., Moreno R.D. A decrease of docosahexaenoic acid in testes of mice fed a high-fat diet is associated with impaired sperm acrosome reaction and fertility. Asian J. Androl. 2021;23:306–313. doi: 10.4103/aja.aja_76_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mu Y., Yan W.-J., Yin T.-L., Zhang Y., Li J., Yang J. Diet-induced obesity impairs spermatogenesis: A potential role for autophagy. Sci. Rep. 2017;7:43475. doi: 10.1038/srep43475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francou M.M., Girela J.L., De Juan A., Ten J., Bernabeu R., De Juan J. Human sperm motility, capacitation and acrosome reaction are impaired by 2-arachidonoylglycerol endocannabinoid. Histol. Histopathol. 2017;32:1351–1358. doi: 10.14670/HH-11-911. [DOI] [PubMed] [Google Scholar]
- 19.Miller M.R., Mannowetz N., Iavarone A.T., Safavi R., Gracheva E.O., Smith J.F., Hill R.Z., Bautista D.M., Kirichok Y., Lishko P.V. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science. 2016;352:555–559. doi: 10.1126/science.aad6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xuan X.J., Xu C., Zhao Y.R., Wu K.L., Chen T., Zhang H.B., Li X., Su S.Z., Ma G., Tang R., et al. Application of spontaneous acrosome reaction of sperm in prediction of outcome of in-vitro fertilization and embryo transfer. Zhonghua Yi Xue Za Zhi. 2016;96:1285–1288. doi: 10.3760/cma.j.issn.0376-2491.2016.16.013. [DOI] [PubMed] [Google Scholar]
- 21.Ushiyama A., Tajima A., Ishikawa N., Asano A. Modification of membrane cholesterol and desmosterol in chicken spermatozoa improves post-thaw survival and prevents impairment of sperm function after cryopreservation. Reprod. Fertil. Dev. 2018;30:591–599. doi: 10.1071/RD17076. [DOI] [PubMed] [Google Scholar]
- 22.Kholkute S.D., Meherji P., Puri C.P. Capacitation and the acrosome reaction in sperm from men with various semen profiles monitored by a chlortetracycline fluorescence assay. Int. J. Androl. 1992;15:43–53. doi: 10.1111/j.1365-2605.1992.tb01113.x. [DOI] [PubMed] [Google Scholar]
- 23.Topfer-Petersen E., Volcker C., Heissler E., Schill W.B. Absence of acrosome reaction in polyzoospermia. Andrologia. 1987;19:225–228. doi: 10.1111/j.1439-0272.1987.tb02336.x. [DOI] [PubMed] [Google Scholar]
- 24.Schill W.B., Topfer-Petersen E., Heissler E. The sperm acrosome: Functional and clinical aspects. Hum. Reprod. 1988;3:139–145. doi: 10.1093/oxfordjournals.humrep.a136663. [DOI] [PubMed] [Google Scholar]
- 25.Herrera J., Fierro R., Zayas H., Conejo J., Jimenez I., Garcia A., Betancourt M. Acrosome reaction in fertile and subfertile boar sperm. Arch. Androl. 2002;48:133–139. doi: 10.1080/014850102317267445. [DOI] [PubMed] [Google Scholar]
- 26.Guidobaldi H.A., Hirohashi N., Cubilla M., Buffone M.G., Giojalas L.C. An intact acrosome is required for the chemotactic response to progesterone in mouse spermatozoa. Mol. Reprod. Dev. 2017;84:310–315. doi: 10.1002/mrd.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suarez S.S., Katz D.F., Overstreet J.W. Movement characteristics and acrosomal status of rabbit spermatozoa recovered at the site and time of fertilization. Biol. Reprod. 1983;29:1277–1287. doi: 10.1095/biolreprod29.5.1277. [DOI] [PubMed] [Google Scholar]
- 28.Cohen G., Rubinstein S., Gur Y., Breitbart H. Crosstalk between protein kinase A and C regulates phospholipase D and F-actin formation during sperm capacitation. Dev. Biol. 2004;267:230–241. doi: 10.1016/j.ydbio.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 29.Visconti P.E., Bailey J.L., Moore G.D., Pan D., Olds-Clarke P., Kopf G.S. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- 30.Visconti P.E., Moore G.D., Bailey J.L., Laclerc P., Connors S.A., Pan D., Olds-Clarke P., Kopf G.S. Capacitation in mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995;121:1139–1150. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- 31.Tsirulnikov E., Huta Y., Breitbart H. PKA and PI3K activities during capacitation protect sperm from undergoing spontaneous acrosome reaction. Theriogenology. 2019;128:54–61. doi: 10.1016/j.theriogenology.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 32.Lee M.A., Kopf G.S., Storey B.T. Effects of phorbol esters and a diacylglycerol on the mouse sperm acrosome reaction induced by the zona pellucida. Biol. Reprod. 1987;36:617–627. doi: 10.1095/biolreprod36.3.617. [DOI] [PubMed] [Google Scholar]
- 33.Albrizio M., Lacalandra G.M., Cinone M. The role of bicarbonate in the modulation of capacitation, spontaneous acrosome reaction and motility of equine fresh and frozen spermatozoa. Theriogenology. 2022;187:112–118. doi: 10.1016/j.theriogenology.2022.04.032. [DOI] [PubMed] [Google Scholar]
- 34.Visconti P.E., Stewart-Savage J., Blasco A., Battaglia L., Miranda P., Kopf G.S., Tezon J.G. Roles of bicarbonate, cAMP, and protein tyrosine phosphorylation on capacitation and the spontaneous acrosome reaction of hamster sperm. Biol. Reprod. 1999;61:76–84. doi: 10.1095/biolreprod61.1.76. [DOI] [PubMed] [Google Scholar]
- 35.Green S., Fishel S. Morphology comparison of individually selected hyperactivated and non-hyperactivated human spermatozoa. Hum. Reprod. 1999;14:123–130. doi: 10.1093/humrep/14.1.123. [DOI] [PubMed] [Google Scholar]
- 36.Angeles-Floriano T., Roa-Espitia A.L., Baltierrez-Hoyos R., Cordero-Martinez J., Elizondo G., Hernandez-Gonzalez E.O. Absence of aryl hydrocarbon receptor alters CDC42 expression and prevents actin polymerization during capacitation. Mol. Reprod. Dev. 2016;83:1015–1026. doi: 10.1002/mrd.22736. [DOI] [PubMed] [Google Scholar]
- 37.Finkelstein M., Etkovitz N., Breitbart H. Role and regulation of sperm gelsolin prior to fertilization. J. Biol. Chem. 2010;285:39702–39709. doi: 10.1074/jbc.M110.170951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Etkovitz N., Rubinstein S., Daniel L., Breitbart H. Role of PI3-kinase and PI4-kinase in actin polymerization during bovine sperm capacitation. Biol. Reprod. 2007;77:263–273. doi: 10.1095/biolreprod.106.056705. [DOI] [PubMed] [Google Scholar]
- 39.Rotfeld H., Hillman P., Ickowicz D., Breitbart H. PKA and CaMKII mediate PI3K activation in bovine sperm by inhibition of the PKC/PP1 cascade. Reproduction. 2014;147:347–356. doi: 10.1530/REP-13-0560. [DOI] [PubMed] [Google Scholar]
- 40.Belenky M., Breitbart H. Role and regulation of glycogen synthase kinase-3 beta in bovine spermatozoa. Mol. Reprod. Dev. 2017;84:8–18. doi: 10.1002/mrd.22759. [DOI] [PubMed] [Google Scholar]
- 41.Grinshtain E., Shpungin S., Baum M., Nir U., Breitbart H. The Fer tyrosine kinase protects sperm from spontaneous acrosome reaction. Dev. Biol. 2022;487:24–33. doi: 10.1016/j.ydbio.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Priyadarshana C., Setiawan R., Tajima A., Asano A. Src family kinases-mediated negative regulation of sperm acrosome reaction in chickens (Gallus gallus domesticus) PLoS ONE. 2020;15:e0241181. doi: 10.1371/journal.pone.0241181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caran N., Johnson L.D., Jenkins K.J., Tombes R.M. Cytosolic targeting domains of gamma and delta calmodulin-dependent protein kinase II. J. Biol. Chem. 2001;276:42514–42519. doi: 10.1074/jbc.M103013200. [DOI] [PubMed] [Google Scholar]
- 44.Ackermann F., Zitranski N., Borth H., Buech T., Gudermann T., Boekhoff I. CaMKIIalpha interacts with multi-PDZ domain protein MUPP1 in spermatozoa and prevents spontaneous acrosomal exocytosis. Pt 24J. Cell Sci. 2009;122:4547–4557. doi: 10.1242/jcs.058263. [DOI] [PubMed] [Google Scholar]
- 45.Huta Y., Nitzan Y., Breitbart H. Ezrin protects bovine spermatozoa from spontaneous acrosome reaction. Theriogenology. 2020;151:119–127. doi: 10.1016/j.theriogenology.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 46.Stock C.E., Fraser L.R. Divalent cations, capacitation and the acrosome reaction in human spermatozoa. J. Reprod. Fertil. 1989;87:463–478. doi: 10.1530/jrf.0.0870463. [DOI] [PubMed] [Google Scholar]
- 47.Luconi M., Krausz C., Forti G., Baldi E. Extracellular calcium negatively modulates tyrosine phosphorylation and tyrosine kinase activity during capacitation of human spermatozoa. Biol. Reprod. 1996;55:207–216. doi: 10.1095/biolreprod55.1.207. [DOI] [PubMed] [Google Scholar]
- 48.Dahan T., Breitbart H. Involvement of metabolic pathway in the sperm spontaneous acrosome reaction. Theriogenology. 2022;192:38–44. doi: 10.1016/j.theriogenology.2022.08.018. [DOI] [PubMed] [Google Scholar]
- 49.Walensky L.D., Snyder S.H. Inositol 1,4,5-trisphosphate receptors selectively localized to the acrosomes of mammalian sperm. J. Cell Biol. 1995;130:857–869. doi: 10.1083/jcb.130.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Itzhakov D., Nitzan Y., Breitbart H. Protein kinase A inhibition induces EPAC-dependent acrosomal exocytosis in human sperm. Asian J. Androl. 2019;21:337–344. doi: 10.4103/aja.aja_99_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Endo D., Kon S., Sato T., Toyama F., Katsura Y., Nakauchi Y., Takayama-Watanabe E., Watanabe A. NMDA-type glutamate receptors mediate the acrosome reaction and motility initiation in newt sperm. Mol. Reprod. Dev. 2019;86:1106–1115. doi: 10.1002/mrd.23225. [DOI] [PubMed] [Google Scholar]
- 52.Lv M.G., Chen W.Q., Weng S.Q., Chen H.Y., Cheng Y.M., Luo T. Rosmarinic acid compromises human sperm functions by an intracellular Ca2+ concentration-related mechanism. Reprod. Toxicol. 2018;81:58–63. doi: 10.1016/j.reprotox.2018.07.079. [DOI] [PubMed] [Google Scholar]
- 53.Zeng X.-H., Navarro B., Xia X.-M., Clapham D.E., Lingle C.J. Simultaneous knockout of Slo3 and CatSper1 abolishes all alkalization- and voltage-activated current in mouse spermatozoa. J. Gen. Physiol. 2013;142:305–313. doi: 10.1085/jgp.201311011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Navarro B., Kirichok Y., Clapham D.E. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc. Natl. Acad. Sci. USA. 2007;104:7688–7692. doi: 10.1073/pnas.0702018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu S.H., Yen Y.K., Ling T.Y., Cheng K.T., Shu J.A., Au H.K., Huang Y.H. Capacitation suppression by mouse seminal vesicle autoantigen involves a decrease in plasma membrane Ca2+-ATPase (PMCA)-mediated intracellular calcium. J. Cell. Biochem. 2010;111:1188–1198. doi: 10.1002/jcb.22844. [DOI] [PubMed] [Google Scholar]
- 56.Mata-Martinez E., Darszon A., Trevino C.L. pH-dependent Ca2+ oscillations prevent untimely acrosome reaction in human sperm. Biochem. Biophys. Res. Commun. 2018;497:146–152. doi: 10.1016/j.bbrc.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 57.Sanchez-Cardenas C., Servin-Vences M.R., Jose O., Trevino C.L., Hernandez-Cruz A., Darszon A. Acrosome reaction and Ca2+ imaging in single human spermatozoa: New regulatory roles of [Ca2+]i. Biol. Reprod. 2014;91:67. doi: 10.1095/biolreprod.114.119768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koh T.W., Bellen H.J. Synaptotagmin I, a Ca2+ sensor for neurotransmitter release. Trends Neurosci. 2003;26:413–422. doi: 10.1016/S0166-2236(03)00195-4. [DOI] [PubMed] [Google Scholar]
- 59.Brose N. For better or for worse: Complexins regulate SNARE function and vesicle fusion. Traffic. 2008;9:1403–1413. doi: 10.1111/j.1600-0854.2008.00758.x. [DOI] [PubMed] [Google Scholar]
- 60.Brukman N.G., Nunez S.Y., Puga Molina L.D.C., Buffone M.G., Darszon A., Cuasnicu P.S., Da Ros V.G. Tyrosine phosphorylation signaling regulates Ca(2+) entry by affecting intracellular pH during human sperm capacitation. J. Cell. Physiol. 2019;234:5276–5288. doi: 10.1002/jcp.27337. [DOI] [PubMed] [Google Scholar]
- 61.McMahon H.T., Missler M., Li C., Sudhof T.C. Complexins: Cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83:111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 62.Sudhof T.C., Rothman J.E. Membrane fusion: Grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weber T., Zemelman B.V., McNew J.A., Westermann B., Gmachl M., Parlati F., Sollner T.H., Rothman J.E. SNAREpins: Minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/S0092-8674(00)81404-X. [DOI] [PubMed] [Google Scholar]
- 64.Nakanishi T., Ikawa M., Yamada S., Toshimori K., Okabe M. Alkalinization of acrosome measured by GFP as a pH indicator and its relation to sperm capacitation. Dev. Biol. 2001;237:222–231. doi: 10.1006/dbio.2001.0353. [DOI] [PubMed] [Google Scholar]
- 65.Lishko P.V., Kirichok Y. The role of Hv1 and CatSper channels in sperm activation. J. Physiol. 2010;588:4667–4672. doi: 10.1113/jphysiol.2010.194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barati E., Nikzad H., Karimian M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020;77:93–113. doi: 10.1007/s00018-019-03253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Flaherty C., de Lamirande E., Gagnon C. Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radic. Biol. Med. 2006;40:1045–1055. doi: 10.1016/j.freeradbiomed.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 68.Bui A.D., Sharma R., Henkel R., Agarwal A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia. 2018;50:e13012. doi: 10.1111/and.13012. [DOI] [PubMed] [Google Scholar]
- 69.Alvarez J.G., Touchtone J.C., Blasco L., Storey B.T. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa: Superoxide dismutase as a major enzyme protectant against oxygen toxicity. J. Androl. 1987;8:338–348. doi: 10.1002/j.1939-4640.1987.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 70.Jeulin C., Soufir J.C., Weber P., Laval-Martin D., Calvayrac R. Catalase activity in human spermatozoa and seminal plasma. Gamete Res. 1989;24:185–196. doi: 10.1002/mrd.1120240206. [DOI] [PubMed] [Google Scholar]
- 71.Nir U., Grinshtain E., Breitbart H. Fer and FerT: A New Regulatory Link between Sperm and Cancer Cells. Int. J. Mol. Sci. 2023;24:5256. doi: 10.3390/ijms24065256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Lamirande E., Gagnon C. A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int. J. Androl. 1993;16:21–25. doi: 10.1111/j.1365-2605.1993.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 73.Rivlin J., Mendel J., Rubinstein S., Etkovitz N., Breitbart H. Role of hydrogen peroxide in sperm capacitation and acrosome reaction. Biol. Reprod. 2004;70:518–522. doi: 10.1095/biolreprod.103.020487. [DOI] [PubMed] [Google Scholar]
- 74.de Lamirande E., O’Flaherty C. Sperm activation: Role of reactive oxygen species and kinases. Biochim. Biophys. Acta. 2008;1784:106–115. doi: 10.1016/j.bbapap.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 75.Faggi M., Vanzetti A., Teijeiro J.M. Effect of glucose and reactive oxygen species on boar sperm induced-acrosome exocytosis. Res. Vet. Sci. 2023;164:105013. doi: 10.1016/j.rvsc.2023.105013. [DOI] [PubMed] [Google Scholar]
- 76.Yaffe E., Hikri E., Elkis Y., Cohen O., Segal A., Makovski A., Varvak A., Shpungin S., Nir U. Oncogenic properties of a spermatogenic meiotic variant of fer kinase expressed in somatic cells. Cancer Res. 2014;74:6474–6485. doi: 10.1158/0008-5472.CAN-14-0058. [DOI] [PubMed] [Google Scholar]
- 77.Xiao Y., Wen Z.Z., Wu B., Zhu H.X., Zhang A.Z., Li J.Y., Gao J.G. Deletion of Aldh4a1 Leads to Impaired Sperm Maturation in Mice. Mol. Biol. 2022;56:585–594. doi: 10.1134/S002689332204015X. [DOI] [PubMed] [Google Scholar]
- 78.Choi Y.J., Uhm S.J., Song S.J., Song H., Park J.K., Kim T., Park C., Kim J.H. Cytochrome c upregulation during capacitation and spontaneous acrosome reaction determines the fate of pig sperm cells: Linking proteome analysis. J. Reprod. Dev. 2008;54:68–83. doi: 10.1262/jrd.19116. [DOI] [PubMed] [Google Scholar]
- 79.delBarco-Trillo J., Mateo R., Roldan E.R. Differences in the fatty-acid composition of rodent spermatozoa are associated to levels of sperm competition. Biol. Open. 2015;4:466–473. doi: 10.1242/bio.201411288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li H.L., Liu D.P., Liang C.C. Paraoxonase gene polymorphisms, oxidative stress, and diseases. J. Mol. Med. 2003;81:766–779. doi: 10.1007/s00109-003-0481-4. [DOI] [PubMed] [Google Scholar]
- 81.Efrat M., Stein A., Pinkas H., Breitbart H., Unger R., Birk R. Paraoxonase 1 (PON1) attenuates sperm hyperactivity and spontaneous acrosome reaction. Andrology. 2019;7:24–30. doi: 10.1111/andr.12552. [DOI] [PubMed] [Google Scholar]
- 82.Volk M., Jaklic H., Zorn B., Peterlin B. Association between male infertility and genetic variability at the PON1/2 and GSTM1/T1 gene loci. Reprod. Biomed. Online. 2011;23:105–110. doi: 10.1016/j.rbmo.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 83.Plum L.M., Rink L., Haase H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health. 2010;7:1342–1365. doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sauer A., Hagmeyer S., Grabrucker A. Nutritional Deficiency. IntechOpen; London, UK: 2016. Zinc Deficiency; pp. 23–46. [Google Scholar]
- 85.Wani A., Parveen N., Ansari M.O., Ahmad F., Jameel S., Shadab G.G.H.A. Zinc: An element of extensive medical importance. Curr. Med. Res. Pract. 2017;7:90–98. doi: 10.1016/j.cmrp.2017.02.006. [DOI] [Google Scholar]
- 86.Albert A. Selective Toxicity: The Physico-Chemical Basis of Therapy. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2012. [Google Scholar]
- 87.Vijayalakshmi K., Sivaraj D. Enhanced antibacterial activity of Cr doped ZnO nanorods synthesized using microwave processing. RSC Adv. R. Soc. Chem. 2015;5:68461–68469. doi: 10.1039/C5RA13375K. [DOI] [Google Scholar]
- 88.Colagar A.H., Marzony E.T., Chaichi M.J. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr. Res. 2009;29:82–88. doi: 10.1016/j.nutres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 89.Brito M., Figueroa J., Vera J.C., Cortés P., Hott R., Burzio L.O. Phosphoproteins are structural components of bull sperm outer dense fiber. Gamete Res. 1986;15:327–336. doi: 10.1002/mrd.1120150406. [DOI] [Google Scholar]
- 90.O’Flaherty C., Matsushita-Fournier D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017;97:577–585. doi: 10.1093/biolre/iox104. [DOI] [PubMed] [Google Scholar]
- 91.Lee S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxidative Med. Cell. Longev. 2018;2018:9156285. doi: 10.1155/2018/9156285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kerns K., Sharif M., Zigo M., Xu W., Hamilton L.E., Sutovsky M., Ellersieck M., Drobnis E.Z., Bovin N., Oko R., et al. Sperm Cohort-Specific Zinc Signature Acquisition and Capacitation-Induced Zinc Flux Regulate Sperm-Oviduct and Sperm-Zona Pellucida Interactions. Int. J. Mol. Sci. 2020;21:2121. doi: 10.3390/ijms21062121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clapper D.L., Davis J.A., Lamothe P.J., Patton C., Epel D. Involvement of zinc in the regulation of pHi, motility, and acrosome reactions in sea urchin sperm. J. Cell Biol. 1985;100:1817–1824. doi: 10.1083/jcb.100.6.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Allouche-Fitoussi D., Bakhshi D., Breitbart H. Signaling pathways involved in human sperm hyperactivated motility stimulated by Zn2. Mol. Reprod. Dev. 2018;85:543–556. doi: 10.1002/mrd.22996. [DOI] [PubMed] [Google Scholar]
- 95.Schneider M., Forster H., Boersma A., Seiler A., Wehnes H., Sinowatz F., Neumuller C., Deutsch M.J., Walch A., Hrabe de Angelis M., et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 2009;23:3233–3242. doi: 10.1096/fj.09-132795. [DOI] [PubMed] [Google Scholar]
- 96.Michailov Y., Ickowicz D., Breitbart H. Zn2+-stimulation of sperm capacitation and of the acrosome reaction is mediated by EGFR activation. Dev. Biol. 2014;396:246–255. doi: 10.1016/j.ydbio.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 97.Etkovitz N., Tirosh Y., Chazan R., Jaldety Y., Daniel L., Rubinstein S., Breitbart H. Bovine sperm acrosome reaction induced by G-protein-coupled receptor agonists is mediated by epidermal growth factor receptor transactivation. Dev. Biol. 2009;334:447–457. doi: 10.1016/j.ydbio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 98.Windler W., Bönigk W., Körschen H.G., Grahn E., Strünker T., Seifert R., Kaupp U.B. The solute carrier SLC9C1 is a Na+/H+-exchanger gated by an S4-type voltage-sensor and cyclic-nucleotide binding. Nat. Commun. 2018;9:2809. doi: 10.1038/s41467-018-05253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ho H.C., Suarez S.S. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca2+ store is involved in regulating sperm hyperactivated motility. Biol. Reprod. 2001;65:1606–1615. doi: 10.1095/biolreprod65.5.1606. [DOI] [PubMed] [Google Scholar]
- 100.Bowker Z., Goldstein S., Breitbart H. Protein acetylation protects sperm from spontaneous acrosome reaction. Theriogenology. 2022;191:231–238. doi: 10.1016/j.theriogenology.2022.08.005. [DOI] [PubMed] [Google Scholar]
- 101.Anderson K.A., Hirschey M.D. Mitochondrial protein acetylation regulates metabolism. Essays Biochem. 2012;52:23–35. doi: 10.1042/bse0520023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun G., Jiang M., Zhou T., Guo Y., Cui Y., Guo X., Sha J. Insights into the lysine acetylproteome of human sperm. J. Proteom. 2014;109:199–211. doi: 10.1016/j.jprot.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 103.Yu H., Diao H., Wang C., Lin Y., Yu F., Lu H., Xu W., Li Z., Shi H., Zhao S., et al. Acetylproteomic analysis reveals functional implications of lysine acetylation in human spermatozoa (sperm) Mol. Cell. Proteom. 2015;14:1009–1023. doi: 10.1074/mcp.M114.041384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Piperno G., Fuller M.T. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J. Cell Biol. 1985;101:2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhagwat S., Dalvi V., Chandrasekhar D., Matthew T., Acharya K., Gajbhiye R., Kulkarni V., Sonawane S., Ghosalkar M., Parte P. Acetylated alpha-tubulin is reduced in individuals with poor sperm motility. Fertil. Steril. 2014;101:95–104.e3. doi: 10.1016/j.fertnstert.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 106.Ritagliati C., Luque G.M., Stival C., Baro Graf C., Buffone M.G., Krapf D. Lysine acetylation modulates mouse sperm capacitation. Sci. Rep. 2018;8:13334. doi: 10.1038/s41598-018-31557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Branham M.T., Bustos M.A., De Blas G.A., Rehmann H., Zarelli V.E., Trevino C.L., Darszon A., Mayorga L.S., Tomes C.N. Epac activates the small G proteins Rap1 and Rab3A to achieve exocytosis. J. Biol. Chem. 2009;284:24825–24839. doi: 10.1074/jbc.M109.015362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Branham M.T., Mayorga L.S., Tomes C.N. Calcium-induced acrosomal exocytosis requires cAMP acting through a protein kinase A-independent, Epac-mediated pathway. J. Biol. Chem. 2006;281:8656–8666. doi: 10.1074/jbc.M508854200. [DOI] [PubMed] [Google Scholar]
- 109.Azoulay Y., Malik Z., Breitbart H. Sperm interaction with bacteria induces the spontaneous acrosome reaction. Theriogenology. 2023;203:82–88. doi: 10.1016/j.theriogenology.2023.02.029. [DOI] [PubMed] [Google Scholar]
- 110.Zhou Y.S., Webb S., Lettice L., Tardif S., Kilanowski F., Tyrrell C., Macpherson H., Semple F., Tennant P., Baker T., et al. Partial deletion of chromosome 8 beta-defensin cluster confers sperm dysfunction and infertility in male mice. PLoS Genet. 2013;9:e1003826. doi: 10.1371/journal.pgen.1003826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dorin J.R. Novel phenotype of mouse spermatozoa following deletion of nine beta-defensin genes. Asian J. Androl. 2015;17:716–719. doi: 10.4103/1008-682X.159712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sebkova N., Ded L., Vesela K., Dvorakova-Hortova K. Progress of sperm IZUMO1 relocation during spontaneous acrosome reaction. Reproduction. 2014;147:231–240. doi: 10.1530/REP-13-0193. [DOI] [PubMed] [Google Scholar]
- 113.Inoue N., Ikawa M., Nakanishi T., Matsumoto M., Nomura M., Seya T., Okabe M. Disruption of mouse CD46 causes an accelerated spontaneous acrosome reaction in sperm. Mol. Cell. Biol. 2003;23:2614–2622. doi: 10.1128/MCB.23.7.2614-2622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johnson P.M., Clift L.E., Andrlikova P., Jursova M., Flanagan B.F., Cummerson J.A., Stopka P., Dvorakova-Hortova K. Rapid sperm acrosome reaction in the absence of acrosomal CD46 expression in promiscuous field mice (Apodemus) Reproduction. 2007;134:739–747. doi: 10.1530/REP-07-0363. [DOI] [PubMed] [Google Scholar]
- 115.Clift L.E., Andrlikova P., Frolikova M., Stopka P., Bryja J., Flanagan B.F., Johnson P.M., Dvorakova-Hortova K. Absence of spermatozoal CD46 protein expression and associated rapid acrosome reaction rate in striped field mice (Apodemus agrarius) Reprod. Biol. Endocrinol. 2009;7:29. doi: 10.1186/1477-7827-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wen Z., Liu D., Zhu H., Sun X., Xiao Y., Lin Z., Zhang A., Ye C., Gao J. Deficiency for Lcn8 causes epididymal sperm maturation defects in mice. Biochem. Biophys. Res. Commun. 2021;548:7–13. doi: 10.1016/j.bbrc.2021.02.052. [DOI] [PubMed] [Google Scholar]
- 117.Jaldety Y., Glick Y., Orr-Urtreger A., Ickowicz D., Gerber D., Breitbart H. Sperm epidermal growth factor receptor (EGFR) mediates alpha7 acetylcholine receptor (AChR) activation to promote fertilization. J. Biol. Chem. 2012;287:22328–22340. doi: 10.1074/jbc.M111.292292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Makino Y., Hiradate Y., Umezu K., Hara K., Tanemura K. Expression and Possible Role of Nicotinic Acetylcholine Receptor epsilon Subunit (AChRe) in Mouse Sperm. Biology. 2021;10:46. doi: 10.3390/biology10010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oyeyipo I.P., Maartens P.J., du Plessis S.S. In vitro effects of nicotine on human spermatozoa. Andrologia. 2014;46:887–892. doi: 10.1111/and.12169. [DOI] [PubMed] [Google Scholar]