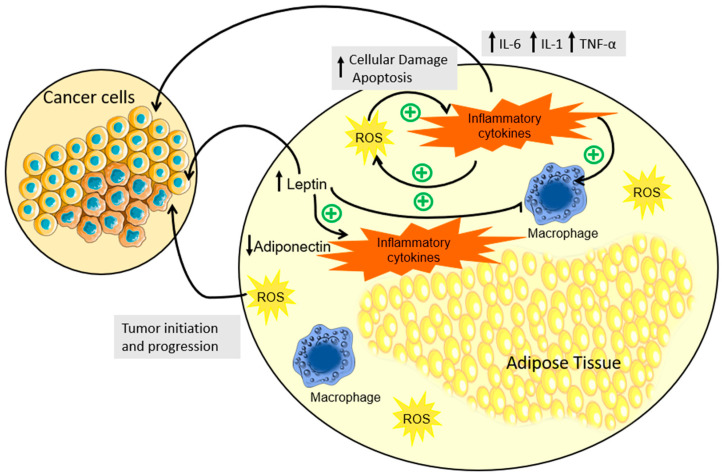
Figure 1.
Schematic representation of inflammation, hormonal dysregulation, and OS in the adipose tissue due to obesity. In individuals with obesity, a notable expansion of adipose tissue triggers an aberrant production and secretion of cytokines, accompanied by the disruption of adipokine regulation. This cascade instigates a series of interconnected events: cytokines foster heightened ROS production, inciting apoptosis, which then exacerbates cytokine release, perpetuating a self-perpetuating cycle. This cytokine orchestration not only contributes to the perpetuation of low-grade chronic inflammation but also significantly augments the landscape for tumor development. Concurrently, elevated leptin levels in obesity correlate with heightened inflammatory cytokine levels, fostering an environment conducive to both the initiation and progression of tumors. In contrast, the diminished presence of adiponectin compounds the scenario, offering a conducive milieu for tumor development. In summary, the complex interplay between obesity, cytokine dynamics, and adipokine regulation unveils a multifaceted process that intricately contributes to chronic inflammation and the initiation and advancement of tumorigenesis. (↓) downregulation; (↑) upregulation; (+) promotion.