Figure 4.
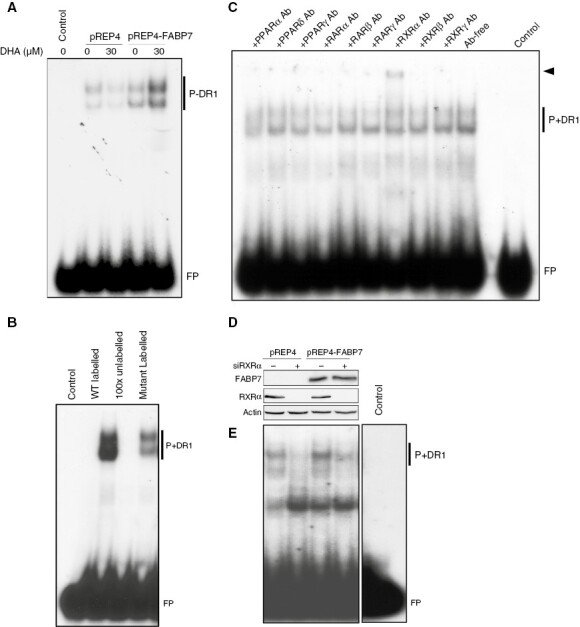
Fatty acid-binding protein 7 (FABP7) facilitates PUFA-induced activation of RXRα. (A) Gel shift showing that DHA treatment enhances formation of nuclear protein-DR1 complexes (P+DR1) in U87 pREP4-FABP7, but not in control cells (pREP4). (B) Inclusion of excess unlabeled PPRE probe (100X) in gel shifts effectively competed with the radio-labeled probe, resulting in undetectable protein-DR1 complexes (P+DR1). Gel shifts with a radio-labeled mutant DR1 probe considerably reduced protein-DR1 complex formation. (C) Supershift assay with antibodies against nine nuclear receptors shows a supershifted band (arrowhead) exclusively in the presence of anti-RXRα antibody. (D) Western blot showing knockdown of RXRα in stable U87 transfectants. (E) RXRα depletion resulted in reduced protein-DR1 complex formation in both U87 pREP4 and U87 pREP4-FABP7 transfectants. Each gene shift experiment was carried out 2 to 4 times. FP denotes free probe. Ab, antibody.
