Fig. 1. G6PD dimer structure showing Arg219 interactions and circular dichroism spectra of recombinant wild-type and Arg219Gly G6PD.
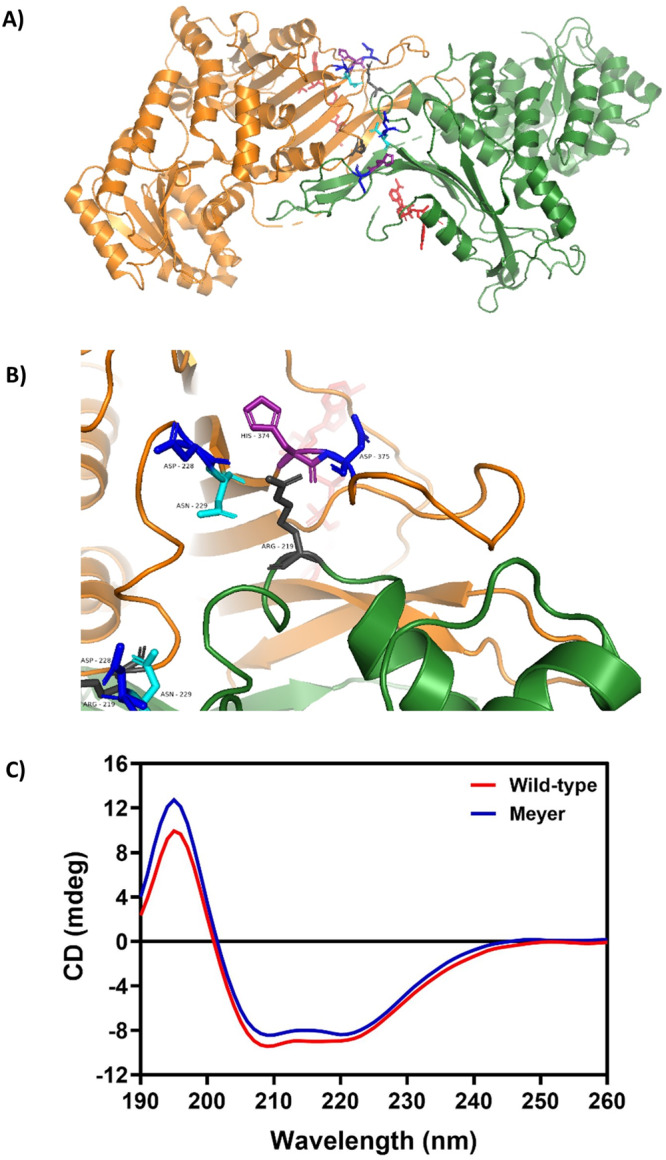
A PyMOL illustration of wild-type G6PD dimer structure adapted from the dimer of dimers structure7, showing Arg219 in gray and relevant interacting residues at the dimer interface: Asp228 and 375 in blue, Asn229 in cyan, and His 374 in purple. Structural NAPD+ is shown in red. B Close-up view of the dimer. Arg219 (center) interacts via its N ɛ and N η1 (right) with the backbone carboxyl O of His374 and Asp375. N ɛ is also proximal to the Asn229 sidechain. Arg N η2 (left) interacts with the backbone carboxyl O of Asp228 and Asn229, as well as the side chain atoms of Asn229. C Far ultraviolet CD spectra of recombinant human G6PD wild-type and Arg219Gly mutant. The protein concentration was 0.15 mg/ml and CD spectra were recorded between 190 and 260 nm at a scan rate of 50 nm/min using a Jasco spectrometer, model J-815.
