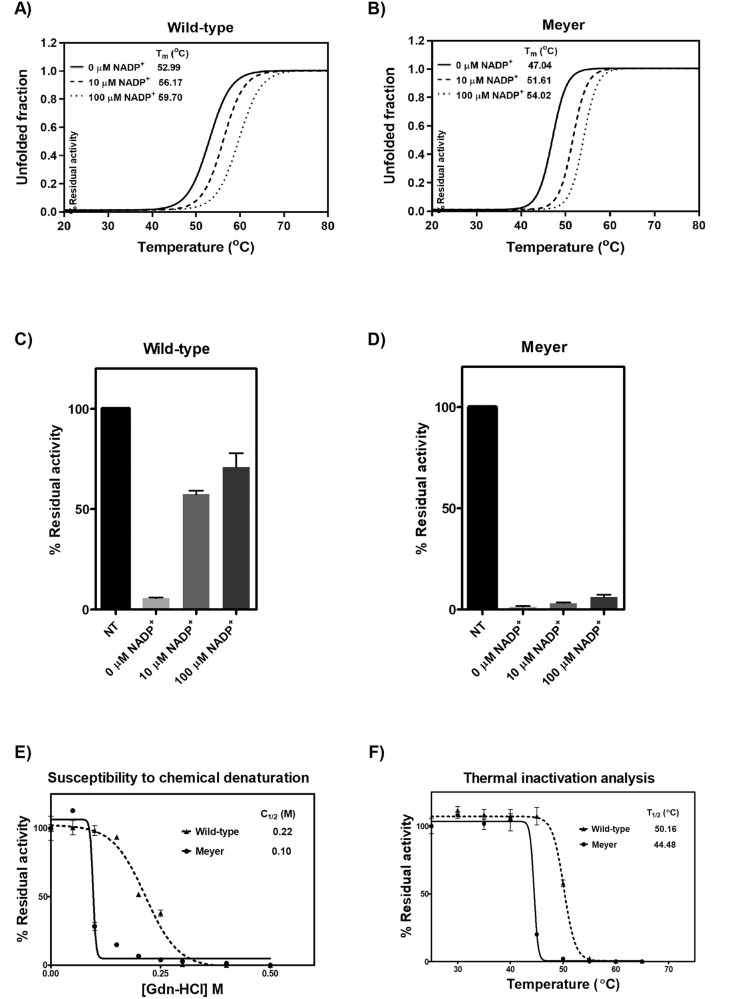
Fig. 2. Structural stability and activity analysis of recombinant wild-type and Arg219Gly G6PD.
Thermal stability analysis of recombinant human (A) G6PD WT and (B) G6PD Meyer. Proteins were heated in the presence of 5x SYPRO Orange reporter dye and various NADP+ concentrations (0, 10, and 100 μM). Protein unfolding was monitored by following the emission of fluorescence dye at 580 nm. Susceptibility to trypsin digestion of recombinant human (C) G6PD WT and (D) G6PD Meyer. Enzyme activity was measured after incubation with 0.5 mg/ml trypsin in the presence of various NADP+ concentrations (0, 10, and 100 μM) at 25 °C for 5 min. Residual enzyme activity is expressed as a percentage of the activity for the same enzyme in the absence of trypsin. NT; no treatment. E Stability analysis of recombinant human G6PD variants upon Gdn-HCl treatment. Enzymatic activity was measured after incubation with various concentrations of Gdn-HCl for 2 h at 37 °C. C1/2 is the Gdn-HCl concentration at which the enzyme loses 50% of its activity. F Thermal inactivation analysis of recombinant human G6PD variants. Enzyme activity was measured after the protein was heated for 20 min. T1/2 is the temperature at which the enzyme loses 50% of its activity. Experiments were performed in triplicate, and data are presented as mean ± SD.