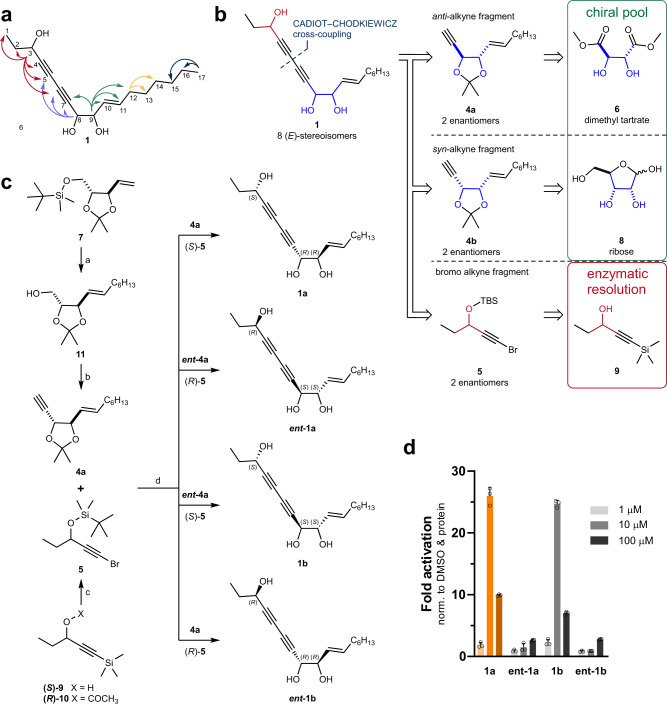
Fig. 2. Structure elucidation of isofalcarintriol by asymmetric synthesis.
a Selected 1H,13C-HMBC correlations observed in isofalcarintriol (1). b Retrosynthetic analysis of isofalcarintriol (1) tracing back to chiral pool starting materials 6, 8 and previously reported enantiopure propargylic alcohol 9. c Synthesis scheme of all four syn-1,2-diol containing isofalcarintriol (1a, b) stereoisomers. Reagents and conditions: a, 1: 1-octene, Grubbs Catalyst 2nd Generation catalytic (cat.), CH2Cl2, 40 °C; 2: tetra-n-butylammonium fluoride, THF, 0 °C to RT, 64–71% over two steps. b, 1: Dess–Martin periodinane (DMP), CH2Cl2, RT; 2: Ohira–Bestmann reagent, K2CO3, MeOH, 0 °C, 72–76% over 2 steps. c, 1: K2CO3, MeOH, 40 °C; 2: t-butyldimethylsilyl chloride (TBSCl), imidazole, CH2Cl2, 0 °C to RT; 3: N-bromosuccinimide, AgNO3 cat., acetone, RT, 38–59% over three steps. d, 1: 4a, CuCl cat., n-BuNH2 (aq.), Et2O, RT, then 5, 0 °C to RT, 61–91%; 2: CF3COOH, THF/water (4:1), 40 °C or HCl (aq.), MeOH, RT, 87–99%.d NRF2 luciferase reporter assay after overnight treatment in transgenic HEK293 cells where only (3 S,8 R,9 R)-isofalcarintriol (1a) and (3 S,8 S,9 S)-isofalcarintriol (1b) activated NRF2, underlining the importance of the configuration of the 3-hydroxy group on activity. Data include three technical replicates and are represented as average + SD. Source data are provided as a Source Data file.