Abstract
The transcription factor p63 is a renowned master regulator of gene expression of stratified epithelia. While multiple proteins have been identified as p63 bona fide targets, little is known about non-coding RNAs (ncRNAs) whose transcription is controlled by p63. Here, we describe a skin-specific non-coding RNA XP33 as a novel target of p63. XP33 levels are increased during keratinocyte differentiation in vitro, while its depletion results in decreased expression of late cornified gene LCE2D. By using publicly available multi-omics data, we show that CTCF and p63 establish an epithelial enhancer to prime XP33 transcription in a tissue-restricted manner. XP33 promoter and enhancer form a chromatin loop exclusively in keratinocytes but not in other cell types. Moreover, the XP33 enhancer is occupied by differentiation-specific factors that control XP33 transcription. Altogether, we identify a tissue-specific non-coding RNA whose expression is epigenetically regulated by p63 and CTCF.
Subject terms: Chromatin remodelling, Transcriptional regulatory elements
Introduction
Being the largest organ, the skin protects the human body from physical, mechanical and chemical traumas, as well as prevents it from infection and dehydration. Starting from single epidermal stem cells, human keratinocytes proliferate within a basal layer and then differentiate into specialised cells which move upwards. The dead cells of the upper layers of the epidermis form so-called cornified envelope harbouring a plethora of hydrophobic protein complexes which allow the skin to function as a barrier [1].
Epidermal differentiation is an extremely complex and exquisitely orchestrated process involving a myriad of players at epigenetic, transcriptional, protein [2] and metabolic [3] levels. Perturbation of the differentiation programme in epidermis or other stratified epithelia leads to the development of pathologies. Delay of cellular differentiation or dedifferentiation is also considered a hallmark of cancers [4–6]. For instance, in head and neck squamous cell carcinoma aberrations of genes regulating proliferation and squamous differentiation contribute to squamous cell carcinogenesis [7–13]. In vitro, induction of keratinocyte differentiation leads to dramatic epigenetic changes and reorganisation of chromatin structure. Early attempts to describe human transcriptome revealed the existence of a plethora of non-coding RNAs (ncRNAs) of unknown function. In recent years, lncRNAs have been shown to allow a fine-tuning of complex tissue- and spatiotemporal processes within the human body such as development or response to external stimuli. In fact, many ncRNAs are expressed at low levels only in determined temporal windows within specific cell types. ncRNA is also deregulated in epithelial diseases, including epithelial tumours [14–20]. ncRNA are found both in the nucleus and cytoplasm and are involved in a broad range of interactions with protein complexes and chromatin. Importantly, we and others have described several long ncRNAs (lncRNAs) including uc.291 [21, 22] and TINCR [23] implemented in terminal differentiation of keratinocytes by directly interacting with chromatin remodellers.
p63 is a master gene regulating the fate of epithelial cells [24–27] and a few other tissues such as the ovary [28]. Being a transcription factor, p63 controls the expression of lineage-specific genes and directs multiple processes including proliferation and differentiation of epithelial cells. Genome-wide studies of the p63 binding landscape unveiled its essential role in establishing lineage-restricted enhancers [29]. p63 interacts with chromatin modellers to increase chromatin accessibility at epithelial enhancers. Moreover, p63 co-operates with insulator CTCF which enables the formation of three-dimensional loops between lineage-specific enhancers and promoters of target genes [30]. While multiple proteins involved in epidermal differentiation have been described as p63 targets genes, little is known about lncRNAs regulated by this transcription factor.
Here, we analyse the expression of lncRNAs in differentiating keratinocytes lacking p63. We identify XP33, a skin-specific lncRNA induced at the late stages of epidermal differentiation in vitro. XP33 expression is regulated by an epithelial enhancer located downstream LCE2 locus. We use publicly available multi-omics data and show that this enhancer is constitutively bound by CTCF but primed by p63 only in epithelial cells. We show that the XP33 promoter and LCE2 enhancer form a loop in keratinocytes but not in other types of epithelial cells, possibly maintained by epithelial transcription factor ZNF750, KLF4 and EGR3.
Results
Expression of skin-specific ncRNA XP33 is affected by p63 depletion
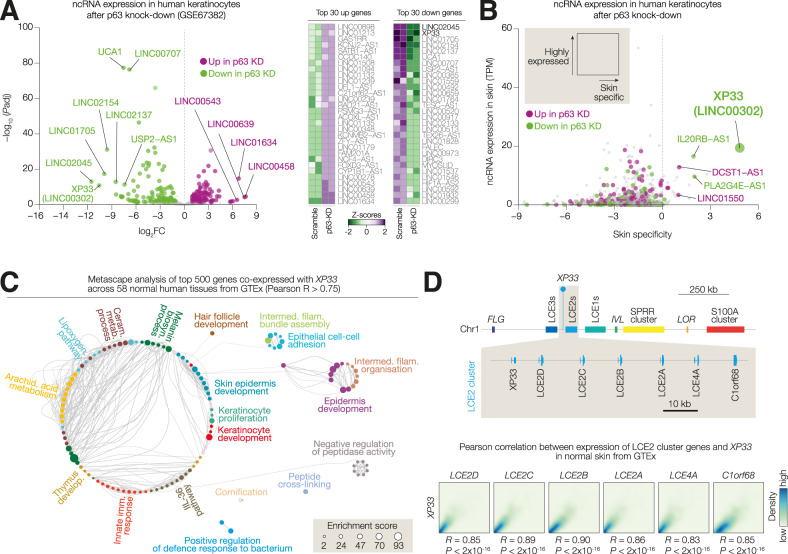
In order to identify lncRNAs differentially expressed in keratinocytes lacking p63, we retrieved lncRNA gene expression values from publicly available RNA sequencing data [31] carried out in differentiated keratinocytes after p63 knock-down (Fig. 1A). We identified 125 down- and 134 upregulated lncRNAs in p63-KD cells compared to scramble control (abs(logFC)>1 and P < 0.05). Of note, we observed a stronger significant down-regulation of lncRNA expression (up to log(FC) = −12) with LINC02045 and XP33 (LINC00302) being the most down-regulated lncRNAs (Fig. 1A, heatmap). We then assessed the abundance of that lncRNA in the skin as well as the specificity of expression in the skin by analysing their expression in the skin versus other human tissues in GTEx (Fig. 1B and Table S3). XP33 was the most skin-specific (skin-specificity score >5) lncRNA expressed in skin (expression >10 TPM) p63-regulated gene. Accordingly, XP33 expression was barely detectable in any tissues other than the skin (Supplementary Fig. S1A). To get insights into the XP33 expression pattern, we identified 500 top genes co-expressed with XP33 by calculating the Pearson correlation coefficient (R) between XP33 expression and the expression of ~57.000 genes across 58 human tissues from GTEx. Metascape analysis revealed that these genes mainly belong to epidermis development pathways (Fig. 1C). XP33 gene is located on chromosome 1 within the epidermal differential complex, containing several clusters of genes essential for terminal differentiation of keratinocytes (Fig. 1D). Due to XP33 location adjacent to LCE2D gene, we hypothesised that XP33 was part of the LCE2 cluster. In fact, its expression is highly correlated (R > 0.85) with an expression of other genes within the LCE2 cluster (LCE2A/B/C/D, LCE4A and C1orf68) (Fig. 1D).
Fig. 1. Expression of skin-specific ncRNA XP33 is affected by p63 depletion.
A Volcano plot showing modulated ncRNAs after p63 knock-down in differentiated keratinocytes. The top 30 down- and upregulated ncRNAs are shown in a heatmap on the right. B Dot plot showing the distribution of the ncRNAs from (A) based on the expression in the skin (in TPM) and skin-specificity score. C Metascape analysis of the top 500 genes co-expressed with XP33 across human tissues from GTEx. D (Top) Schematic showing the genomic location of XP33 within the LCE2 locus on human Chromosome 1. (Bottom) Correlation plots show a correlation between expression levels of XP33 and levels of genes from the LCE2 locus in human skin.
Collectively, we identify XP33 as skin-specific lncRNA whose expression is decreased after p63 knock-down.
XP33 expression is upregulated during induced keratinocyte differentiation
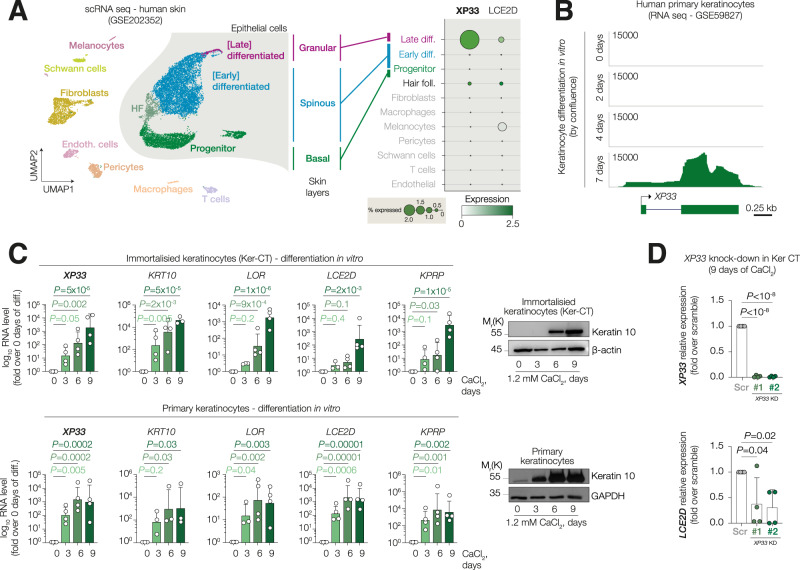
Bulk RNA-seq analysis of gene expression in skin from GTEx only shows average expression of the whole tissue, therefore, to understand which types of skin cells express XP33, we performed de novo analysis of previously published scRNA-seq data (Fig. 2A). By clustering cells using UMAP and annotating clusters with SingleR packages, we were able to detect major epithelial and non-epithelial cell types. XP33 was detectable only in a handful of cells from late differentiation cluster corresponding to upper-spinous and granular layers of the epidermis, while its expression was hardly detectable in progenitor keratinocytes or any other non-epithelial cells in contrast to previous report [32]. Of note, the XP33 expression pattern was similar to its genomic neighbour LCE2D (Fig. 2A). To investigate XP33 behaviour during keratinocyte differentiation in vitro, we analysed XP33 levels in a publicly available RNA sequencing of confluence-induced differentiation of primary human keratinocytes and we saw an up-regulation of this transcript at 7 days of differentiation while in progenitor cells it was barely detectable (Fig. 2B). We then confirmed this observation carrying out the differentiation of both primary and immortalised keratinocytes (Ker-CT) induced by treatment with 1.2 mM calcium chloride (Fig. 2C). Accordingly, XP33 levels increased significantly after 6 days of differentiation (up to 1000-fold) similar to late cornified genes LOR, LCE2D and KPRP, while expression of an early marker keratin 10 was strongly upregulated already after 3 days of calcium treatment. XP33 role has been previously discussed in dermal fibroblasts [32], however, it remains unclear in keratinocytes. Therefore, we knocked-down XP33 expression in Ker-CT cells using two distinct small-interfering RNAs (siRNAs) and induced the cells to differentiate for 9 days (Fig. 2D). Since many lncRNAs are known to regulate the expression of nearby genes, we assessed the expression of its neighbour LCE2D. Interestingly, after XP33 knock-down, LCE2D levels were significantly reduced.
Fig. 2. XP33 expression is upregulated during induced keratinocyte differentiation.
A (Left) UMAP plot showing clusters of cells from human skin. (Right) Dot plot showing expression of XP33 and LCE2D in identified clusters. B RNA-seq signal tracks showing XP33 expression in human keratinocytes induced for differentiation for 2, 4 or 7 days. C qPCR analysis of XP33, KRT10, LCE2D, LOR, and KPRP expression in immortalised cells Ker-CT (top) or primary keratinocytes (bottom). Western blot confirms induction of differentiation by Keratin 10 staining. n = 4 (biological replicates), P by one-way ANOVA. D qPCR analysis of XP33 and LCE2D expression in Ker-CT silenced for XP33 and induced to differentiate for 9 days. n = 4 (biological replicates), P by one-way ANOVA.
Altogether, these data demonstrate that XP33 is expressed during the late stages of epidermal differentiation and it may function to promote the expression of neighbouring genes like LCE2D.
An epithelia-specific enhancer within the LCE2 locus is activated during differentiation
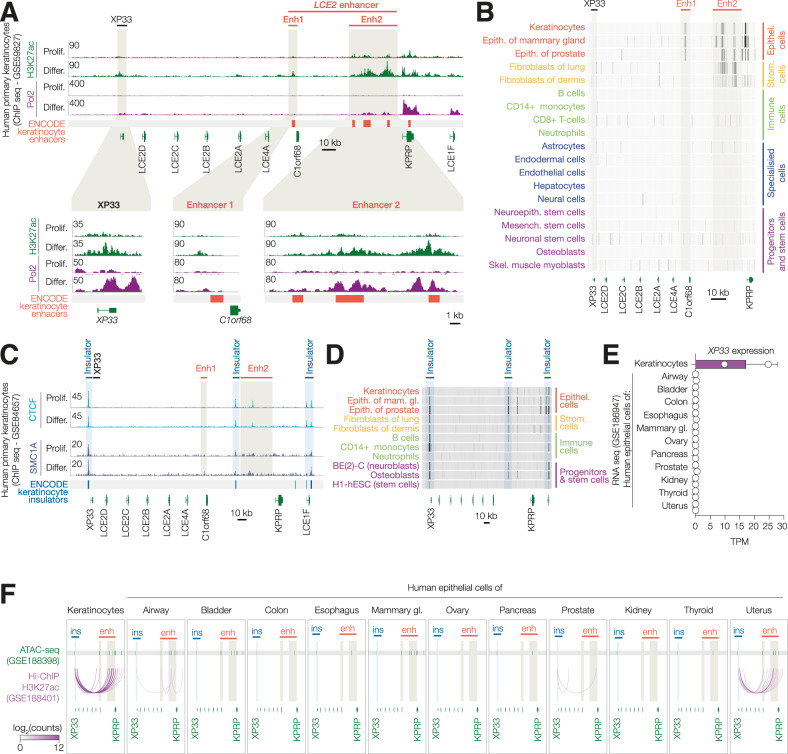
To study the regulation of XP33 expression, we analysed the chromatin state of the LCE2 locus on chromosome 1 using publicly available ChIP-sequencing data (Fig. 3A). We observed an increase in H3K27 acetylation at XP33 and C1orf68 promoters accompanied by an increase of Pol2 occupancy in differentiated keratinocytes, which is consistent with the notion that XP33 is only expressed during late stages of differentiation. Furthermore, a region adjacent to the C1orf68 gene and a large region between C1orf68 and KRPR genes had multiple sites of H3K27ac present already in progenitor cells with a further increase in differentiated keratinocytes. Both regions overlapped with predicted ENCODE keratinocyte enhancers (hereinafter, LCE2 enhancer). Of note, we were able to detect an increase of Pol2 occupancy at LCE2 enhancer in differentiated cells. Analysis of ENCODE ChIP-seq data across multiple tissues revealed the presence of the H3K27ac mark within the LCE2 enhancer region only in epithelial cells of the skin, mammary gland and prostate, but not in other types of cells including immune cells, soft tissue cells or stem cells (Fig. 3B). This suggests that LCE2 enhancer is epithelia-specific.
Fig. 3. An epithelia-specific enhancer within the LCE2 locus is activated during differentiation.
A ChIP-seq tracks of H3K27ac and Pol2 in keratinocytes (proliferating or differentiated). Enlarged areas of XP33 promoter, Enhacer 1 and Enhacer 2 are shown below. B Dense ChIP-seq tracks of H3K27ac in different cell types from ENCODE. C ChIP-seq tracks of CTCF and SMC1A in keratinocytes (proliferating or differentiated). D Dense ChIP-seq tracks of CTCF in different cell types from ENCODE. E XP33 expression from RNA-seq in cells derived from different types of epithelia. F ATAC-seq regions (top) and H3K27ac-HiChIP chromatin loops (bottom) in the same samples.
It has been established that lineage-specific enhancers can regulate the expression of specialised clusters of genes by physically interacting with their promoters [33]. Distal regulatory elements can interact via the formation of chromatin loops maintained by an insulator protein CTCF and cohesin complex. We investigated CTCF occupancy in human keratinocytes and found two distinct sites bound by CTCF flanking LCE2 enhancer and one CTCF site upstream XP33. Furthermore, these regions were also occupied by the SMC1A subunit of the cohesin complex (Fig. 3C). CTCF and cohesin occupancy did not change during differentiation. In contrast to the H3K27ac mark, we saw CTCF binding at identified insulator regions in virtually all cell types analysed, including human embryonic stem cells H1-hESC (Fig. 3D).
To further investigate a three-dimensional structure of XP33-LCE2 cluster in keratinocytes, we simultaneously assessed the expression of XP33, chromatin accessibility and chromatin looping in LCE2 locus across 15 types of epithelial cells including keratinocytes using publicly available data from a recent study [34]. As expected, XP33 was found expressed only in keratinocytes but not in any other epithelial cell types analysed (Fig. 3E). Interestingly, LCE2 enhancer was accessible in a few types of cells, including lung, bladder, mammary gland epithelial cells similar to keratinocytes (Fig. 3F, the upper part—ATAC-seq). Nonetheless, H3K27ac-HiChIP revealed XP33-enhancer looping only in keratinocytes and to a lesser extent in epithelial cells of the uterus, but not other tissues (Fig. 3F, lower part—HiChIP-seq). These findings might explain why XP33 expression is restricted to keratinocytes, while LCE2 enhancer is active and accessible in multiple epithelial cell types.
Collectively, our data suggest that XP33 expression is regulated via promoter-enhancer interaction which is specific to keratinocytes.
p63 is a pioneer factor essential for the establishment of LCE2 enhancer
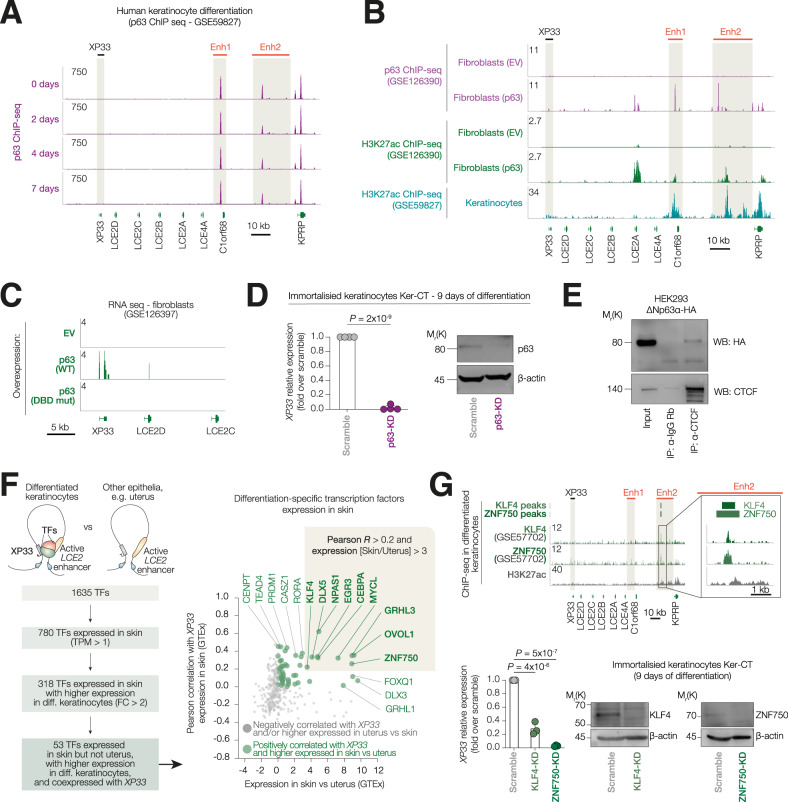
Since we identified XP33 as a p63-regulated gene and considered a pivotal role of p63 in the establishment of epidermal enhancers, we questioned whether the LCE2 enhancer was occupied by p63 (Fig. 4A). We found several sites bound by p63 within LCE2 enhancer, both at enhancer 1 (adjacent to C1orf68) and large enhancer 2 (between C1orf68 and KPRP). p63 binding to LCE2 enhancer did not change during differentiation suggesting that p63 could epigenetically regulate this region already in progenitor cells.
Fig. 4. p63 is a pioneer factor essential for the establishment of an LCE2 enhancer.
A ChIP-seq signal tracks for p63 in human keratinocytes induced for 2, 4 or 7 days of differentiation. B ChIP-seq signal tracks for p63 in H3K27ac in human fibroblasts expressing either empty vector or ΔNp63α. At the bottom, ChIP-seq signal tracks for H3K27ac in human fibroblasts keratinocytes. C RNA-seq signal track of fibroblast expressing either empty vector, or wild-type ΔNp63α, or DND-binding domain mutant of ΔNp63α. D qPCR analysis of XP33 expression in Ker-CT was silenced for p63 and induced to differentiate for 9 days. Western blot confirms an efficient knock-down. n = 4 (biological replicates), P by one-way ANOVA. E Co-immunoprecipitation in HEK293 overexpressing ΔNp63α-HA using either anti-CTCF or anti-IgG antibodies. Western blot for HA and CTCF. n = 2 (biological replicates). F (Left) Schematic shows strategy to select differentiation-specific skin-restricted TFs which may regulate the expression of XP33. (Right) Dot plot showing the distribution of human TFs based on the specificity of expression in skin and correlation of their expression with levels of XP33. Top hits are shown in a beige box. G (Top) ChIP-seq signals for KLF4 and ZNF750 in differentiated keratinocytes. The enlarged area of the LCE2 locus is shown on the right. (Bottom) qPCR analysis of XP33 expression in Ker-CT silenced for KLF4 or ZNF750 and induced to differentiate for 9 days. Western blot confirms an efficient knock-down. n = 3 (biological replicates), P by one-way ANOVA.
To understand whether p63 is essential for LCE2 enhancer activation, we investigated both p63 and H3K27ac occupancy in fibroblasts analysing data from a recent study [29] (Fig. 4B). Exogenous p63 bound the same loci in fibroblasts as endogenous p63 in keratinocytes. Moreover, the same loci gained H3K27 acetylation after p63 overexpression. Interestingly, only overexpression of wild-type p63 but not DNA-binding domain mutant of p63, led to a mild activation of XP33 expression in fibroblasts (Fig. 4C). We then analysed impact of p63 loss on XP33 expression in vitro (Fig. 4D). We observed a significant decrease of XP33 expression in differentiated (9 days) keratinocytes Ker-CT after p63 knock-down. As the LCE2 locus is occupied by CTCF in multiple cell types of non-epithelial origin, we questioned whether p63 could use CTCF as a bait to recognise enhancer regions. p63 was shown to co-operate with CTCF to establish epithelia-specific chromatin architecture [30], even though physical interaction between the two proteins has not been investigated. We, therefore, performed a co-immunoprecipitation of CTCF in HEK293 cell overexpressing ΔNp63α-HA and were able to detect interaction between CTCF and p63.
Since XP33 is specifically expressed in differentiated keratinocytes but not in other epithelial or progenitor cells harbouring an active LCE2 enhancer, we questioned whether there were any specific transcriptional factors (TFs) able to confer XP33 expression in a lineage-specific fashion (Fig. 4F). To address this question, we screened a list of 1635 human TFs based on their expression in skin and a trend for a higher expression in differentiated keratinocytes. We selected 318 TFs and then further screened them according to a co-expression with XP33 in skin (by Pearson correlation) and specificity of expression in the skin over other epithelia harbouring active LCE2 enhancers (e.g., uterus). We identified 9 candidate TFs that could potentially regulate XP33 expression (Fig. 4F). Of note, EGR3 has been already shown to bind LCE2 enhancers [35]. We, therefore, analysed the occupancy of two major TFs, ZNF750 and KLF4, essential for keratinocyte differentiation [36]. In fact, ZNF750 and KLF4 bound a large portion of LCE2 enhancer (Fig. 4G) and their expression was positively correlated with XP33 expression in human skin samples. Furthermore, ZNF750 and KLF4 knock-down in Ker-CT cells resulted in significantly reduced levels of XP33.
Altogether, our analyses show that p63 is a pioneer factor that establishes lineage-specific LCE2 enhancer, while differentiation-specific TFs, like EGR3, ZNF750 and KLF4, may drive XP33 expression in a tissue-restricted manner.
Discussion
Here, we analyse the expression of ncRNAs in differentiated keratinocytes depleted for p63 and identify over 300 modulated non-coding genes. However. most of those genes are expressed at very low levels in the skin or show a higher expression in other tissues, potentially leading to the identification of physiologically irrelevant genes. Given the importance of p63 for epidermal homoeostasis, we only focused our study on p63-regulated ncRNAs specific to the skin. Thus, we identified XP33, also known as long intergenic non-coding RNA 302 (LINC00302), as a skin-restricted and the most down-regulated gene in p63-KD cells. XP33 gene is located on chromosome 1 within the epidermal differentiation complex adjacent to LCE2D gene [37]. XP33 expression in skin is highly correlated with the expression of genes from the LCE2 locus, moreover, the most co-expressed genes for XP33 across human tissues are related to epidermal differentiation pathways. By analysing both primary and immortalised keratinocytes, we showed that XP33 levels drastically increase during in vitro calcium chloride-induced differentiation. Furthermore, single-cell analysis revealed that XP33 expression is restricted to upper-spinous and granular layers of skin. The same trend was observed during confluence-induced differentiation as shown by analysing a previously published RNA-seq. All these observations are in line with the notion that XP33 is a ncNRA related to the terminal differentiation of keratinocytes.
We characterised the chromatin state of the region surrounding the XP33 gene by analysing publicly available ChIP-seq experiments carried out in human keratinocytes. We found two loci resembling active enhancers downstream of XP33 (collectedly called as LCE2 enhancer). Interestingly, LCE2 enhancer shows high levels of H3K27 acetylation in keratinocytes, mammary gland and prostate epithelial cells, but not in other specialised cells, stromal, immune or stem cells. This suggests the presence of an active LCE2 enhancer in distinct epithelia. Nonetheless, XP33 expression is restricted to the skin. This might be explained by the fact that active LCE2 enhancer physically interacts with XP33 region only in keratinocytes but not in other epithelial as shown by analysis of a HiChIP experiment. However, these findings must be confirmed by Hi-C or conventional chromatin conformation capture experiments. Mechanistically, interaction between regulatory elements of chromatin is mediated by an insulator factor CTCF and cohesin complex which establish and maintain three-dimensional chromatin loops. We observed strong CTCF binding both upstream XP33 and within regions flanking LCE2 enhancer. The same sites were co-bound by SMC1A, a component of the cohesin complex. CTCF binding remained unchanged during keratinocyte differentiation in vitro. Furthermore, CTCF binding upstream XP33 promoter was found in multiple cell types, including epithelia, immune, stromal and stem cells. This may suggest that CTCF marks the boundaries of XP33-enhancer loop early during development (Fig. 5), however, due to the closed chromatin within the enhancer region and the absence of specific TFs, this loop remains unstable in most of the cell types.
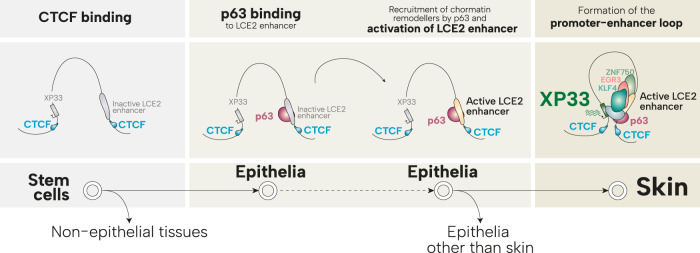
Fig. 5. p63 and CTCF prime an epithelial enhancer to boost expression of XP33.
CTCF binds insulator sites upstream XP33 promoter and within LCE2 enhancer in multiple cells including stem cells. However, only in epithelial cells, p63 activates LCE2 enhancer. Active LCE2 enhancer interacts with XP33 promoter only in differentiating keratinocytes leading to its expression controlled by ZNF750, KLF4 and EGR3.
Similar to CTCF, we found p63 to bind several loci within the LCE2 enhancer and importantly this binding was unchanged during differentiation. It has been speculated that p63 is a pioneer factor that promotes the establishment of epithelial enhancers via direct binding to its motifs on inaccessible chromatin and recruitment of chromatin remodellers. In fact, we observed that overexpression of p63 in stromal cells which normally have LCE2 enhancer in inactive form, led to an increased acetylation of H3K27 within this region (Fig. 5). Moreover, overexpression of p63 in fibroblast was able to induce mild levels of XP33 expression. We demonstrated a physical interaction between p63 and CTCF in vitro, therefore, it is plausible that p63 co-operates with CTCF to establish LCE2 enhancer / XP33 promoter interaction [30]. Undoubtedly, further in vitro experiments are needed to investigate whether loss of p63 or CTCF in keratinocytes leads to closed chromatin conformation of the LCE2 enhancer.
p63 activity in proliferating keratinocytes is necessary to enable XP33 expression during differentiation. Our knock-down experiments confirmed that depletion of p63 led to a dramatic decrease of XP33 at 9 days of differentiation. However, since p63 binding to LCE2 enhancer did not change during differentiation in vitro, it is unlikely that p63 is sufficient to drive XP33 expression during terminal differentiation of keratinocytes. Moreover, in human skin p63 expression is gradually decreased with barely detectable levels of p63 in late spinous layers of the epidermis. One possibility could be that p63 is essential to pre-mark LCE2 enhancer which remains active even when p63 expression begins to decrease in differentiating cells. We hypothesised that other transcription factors, specific to differentiated keratinocytes, might bind LCE2 enhancers to induce XP33 expression during terminal stages of epidermal specialisation. We have screened >1600 human TFs based on their expression in skin, their trend towards an increased expression in differentiated cells, their co-expression with XP33 and skin-restricted expression. We found nine TFs that potentially may regulate XP33 expression. Of note, EGR3 binding to this region has been already described previously [35]. We also found ZNF750 and KLF4, two TFs known to regulate terminal differentiation [36, 38, 39], to bind LCE2 enhancers. Knock-down of both factors significantly reduced levels of XP33 in differentiated cells, suggesting their involvement in the transcription of XP33 and possibly other LCE2 genes (Fig. 5).
Little is known about the function of this lncRNA. XP33 was found to be differentially expressed in psoriasis [40], although its role remains unclear. Our knock-down experiments showed that depletion of XP33 resulted in decreased expression of its genomic neighbour LCE2D which may suggest a cis-acting mechanism of gene expression regulated by XP33, however, this must be investigated more in detail.
In summary, we characterise the lncRNA XP33 and lineage-restricted regulation of its expression. Further work will be needed to understand the function of XP33 in human keratinocytes, epidermal differentiation, and in pathological conditions with loss of differentiated phenotype. Our study also provides a set of other p63-regulated lncRNAs which can be further investigated.
Materials and methods
Cell culture and treatments
Human epidermal keratinocytes (neonatal) were purchased from Lonza (#00192907). Immortalised human keratinocytes Ker-CT were purchased from ATCC (#CRL-4048, RRID:CVCL_S877). Keratinocytes were grown in sub-confluent conditions in EpiLife medium (ThermoFisher, #MEPI500CA) supplemented with growth factors (ThermoFiscer, #S0015) on dishes pre-coated with rat tail collagen type I solution (Corning, #354236). HEK293 cells were grown in Dulbecco’s modified Eagle’s medium (ThermoFisher) supplemented with 10% (vol/vol) fetal bovine serum (ThermoFisher) and 1% penicillin/streptomycin (ThermoFisher). To induce differentiation, keratinocytes were seeded at high confluence and treated with 1.2 mM calcium chloride added directly into the growth medium for indicated times.
RNA interference
RNA interference experiments were performed using Lipofectamine RNAiMAX (ThemoFisher, 13778075) following the manufacturer’s instructions. Cells were seeded onto 12-well plates and transfected with 50 pmol of siRNA and 2.5 µL of the transfection reagent. The day after the first transfection, the cells were induced to differentiate as described before. The transfection was repeated on day 5 of differentiation. Cells were collected at day 9 of differentiation. All siRNAs were purchased from Sigma. siRNA sequences are listed in the Table S1. KLF4 and ZNF750 siRNA sequences were obtained from [41], while TP63 siRNA sequences were obtained from [42]. KLF4 and ZNF750 siRNAs were used as a pool of two siRNAs.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using an RNeasy mini kit (Qiagen, #74004) with DNase I treatment step (Qiagen, #79254) following the manufacturer’s instructions. One microgram of total RNA was used for retrotranscription with SensiFAST cDNA Synthesis Kit (BioLine, # BIO-65054). qPCR on cDNA was performed using PowerUp SYBR Green Master Mix (ThermoFisher, #A25778) in QuantStudio 5 Real-Time PCR System. All samples were run in triplicate. GAPDH was used as house-keeping gene. Differences in gene expression were calculated using the ΔΔCt method. Sequences of the qPCR primers are listed in the Table S1.
Western blot
Keratinocytes were lysed directly on the dish with SDS buffer (100 mM Tris рН 8.8, 1% SDS, 5 mM EDTA, 20 mM DTT, and 2 mM AEBSF). Lysates were resolved in SDS polyacrylamide gel and blotted onto an Amersham Hybond PVDF membrane (GE Healthcare, #GE10600023). Membranes were incubated with primary antibodies overnight at 4 °C. The day after the membranes for washed with 0.1% PBS/Tween-20 three times and incubated with secondary antibodies for 1 h at room temperature. The membranes were washed three times. The signal was revealed using Western Lightning Plus Chemiluminescent Substrate (Revvity, # NEL103E001EA) in the Uvitec Alliance imaging system (Uvitec) or by exposure of X-ray films. The following primary antibodies were used: anti-Keratin-10 (1:1000, BioLegend, #905403, RRID:AB_2749902), anti-GAPDH (1:15000, Sigma, # G8795, RRID:AB_1078991), anti-bet-actin (1:10.000, Sigma, #A5441, RRID:AB_476744), anti-p63alpha (1:500, D2K8X, Cell Signaling, #13109, RRID:AB_2637091), anti-CTCF (D31H2 XP, Cell signaling, #3418, RRID:AB_2086791), anti-HA (BioLegend, #901513, RRID:AB_2565335), anti-KLF4 (R&D systems, AF3640, RRID:AB_2130224), anti-ZNF750 (Sigma, HPA023012-100ul, RRID:AB_1859439), goat anti-mouse IgG (H + L)-HRP conjugate (1:10.000, BioRad, #1706516, RRID:AB_11125547), goat anti-rabbit IgG (H + L)-HRP conjugate (1:10.000, BioRad, #1706515, RRID:AB_11125142), and rabbit anti-rabbit IgG (H + L)-HRP conjugate (1:10.000, BioRad, #1706515, RRID:AB_11125142). Uncropped western blots are shown in Supplementary Fig. S1B.
Co-immunoprecipitation (co-IP)
HEK293 cells were transfected with pcDNA3.1-HA (empty vector, “EV”) or pcDNA3.1-ΔNp63α-HA expressing vectors using Effectene Reagent (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Twenty-four hours after transfection cells were lysed in triton buffer (NaCl 150 mM, Tris HCl pH [5, 7] 50 mM, Triton 0,5%, NaF 50 mM, EDTA [pH 8] 1 mM, 0.1 mM Sodium orthovanadate and cOmplete EDTA-free protease inhibitor cocktail (Merck, #11873580001)). Total protein extract was quantified and 2 mg was used for each co-IP. Lysate pre-clearing was performed by 2 h incubation at 4 °C with 25 µL of Protein A Sepharose 4 Fast Flow (Cytiva, #17528001) diluted in 1 mL of Triton buffer. Two microgram of anti-CTCF (D31H2 XP, Cell signaling, #3418, RRID:AB_2086791) or anti-rabbit IgG isotype Control (Invitrogen, #10500 C, RRID:AB_2532981) antibodies were coated to 25 µL of Protein A Sepharose 4 Fast Flow (Cytiva, #17528001, RRID:AB_2532981) by 2 h incubation at 4 °C in 1 mL of Triton buffer. Co-IP was carried out by overnight incubation of precleared lysates with antibody-coated beads at 4 °C. The day after, beads were washed 6 times in 1 mL of triton buffer, then boiled for 10 min at 98 °C in 2 × 40 µL of Laemmli buffer and analysed by WB.
Publicly available multi-omics data
All the data used in the study are available on GEO NCBI: RNA-seq of human keratinocytes after p63 knock-down (GSE67382) [31], scRNA-seq of human skin (GSE202352) [43], RNA-seq and ChIP-seq for H3K27ac, p63, and Pol2 in human keratinocytes differentiated in vitro (GSE59827) [44], ChIP-seq for CTCF and SMC1A in differentiated human keratinocytes (GSE84657) [33], RNA-seq, ATAC-seq and H3K27ac-HiChIP of epithelia (GSE188405) [34], RNA-seq and ChIP-seq for H3K27ac and p63 in human fibroblasts (GSE126390) [29], ChIP-seq for ZNF750 and KLF4 in differentiated human keratinocytes (GSE57702) [36]. ChIP-seq for H3K27ac and CTCF across different cell types were downloaded from ENCODE Project [45]. Gene expression from normal tissues was downloaded from GTEx Portal [46]. A detailed description of all used resources and datasets, with an overview and limitations is summarised in Table S2.
Bioinformatic analyses
For differential expression of ncRNAs in human keratinocytes after p63 knock-down (GSE67382), raw reads were aligned to the human genome (GENCODE19) and gene expression was quantified using STAR package [47] and filtered for the ncRNAs only. The list of annotated ncRNAs was downloaded from the HUGO database. Differential expression analysis was performed using the DEseq2 package [48]. ncRNAs with abs(log2(FC)) > 1 and P < 0.05 were considered significantly modulated. Skin-specificity scores were calculated as differences between the Z-scores of gene expression in the skin and the maximum value of Z-scores in any other tissues. A positive score indicates higher expression in the skin compared to any other tissue, while a negative score indicates at least one tissue with higher expression compared to the skin. Fold-change values and skin-specificity scores are listed in Table S3. scRNA-seq data were analysed using Seurat package [49]. Briefly, cells with feature numbers > 200 and <2000 and <15% of mitochondrial genes were used for the analysis. Dimensionality reductions were performed using UMAP. Cell types were annotated using SingleR package [50]. Pathway enrichment was performed using Metascape [51]. For the analysis of differentiation-specific TF, the list of known human TF was downloaded from The Human Transcription Factors database [52]. Expression values for the TFs were obtained from the GTEx portal or RNA-seq of keratinocyte differentiation in vitro (GSE59827). ChIP-seq and RNA-seq signal tracks were visualised in the UCSC Genome Browser [53]. Heatmaps were generated with pHeatmap package. Volcano plots and correlation density plots were generated using the ggplot2 package [54].
Statistical analyses
The statistical analyses were performed in GraphPad Prism 9.5.0 (San Diego, USA). For the qPCR analyses, the significance of the differences between the groups was determined by one-way analysis of variance (ANOVA) without adjustments. For the differential gene expression analysis, adjusted P-values were calculated using the DEseq2 package in R.
Supplementary information
Acknowledgements
This work was supported by the Ministry of Health and IDI-IRCCS (RC203), grant A0375-2020-36568 UTV-IDI (to EC) and the Italian Ministry of University and Research under PNRR-CN3 (CN_00000041, CUPE83C22003200001 to EC). AS was supported by REACT-EU PON “Ricerca e Innovazione 2014-2020” (DM 1062/2021).
Author contributions
AS and EC conceived and designed the study. AS carried out bioinformatic analyses and prepared figures. AS, AML, XY, GT and AC performed experiments in vitro. AS wrote the manuscript in consultation with MHC, GM and EC. All authors discussed the results and contributed to the final manuscript.
Data availability
All the high-throughput sequencing data used in this study are publicly available and the accession numbers are listed in Materials and Methods.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/2/2024
The author name Manuela Helmer Citterich has been updated.
Supplementary information
The online version contains supplementary material available at 10.1038/s41420-023-01716-3.
References
- 1.Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- 2.Michaletti A, Mancini M, Smirnov A, Candi E, Melino G, Zolla L. Multi-omics profiling of calcium-induced human keratinocytes differentiation reveals modulation of unfolded protein response signaling pathways. Cell Cycle. 2019;18:2124–40. doi: 10.1080/15384101.2019.1642066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappello A, Mancini M, Madonna S, Rinaldo S, Paone A, Scarponi C, et al. Extracellular serine empowers epidermal proliferation and psoriasis-like symptoms. Sci Adv. 2022;8:eabm7902. doi: 10.1126/sciadv.abm7902. [DOI] [PubMed] [Google Scholar]
- 4.Friedmann-Morvinski D, Verma IM. Dedifferentiation and reprogramming: origins of cancer stem cells. EMBO Rep. 2014;15:244–53. doi: 10.1002/embr.201338254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada Y, Haga H, Yamada Y. Concise review: dedifferentiation meets cancer development: proof of concept for epigenetic cancer. Stem Cells Transl Med. 2014;3:1182–7. doi: 10.5966/sctm.2014-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Unternaehrer JJ. Epithelial-mesenchymal transition and cancer stem cells: at the crossroads of differentiation and dedifferentiation. Dev Dyn. 2019;248:10–20. doi: 10.1002/dvdy.24678. [DOI] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–82. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Qiao P, Zhang J, Chen X, Hu A, Huang S. Crosstalk between ROCK1 and PYROXD1 regulates CAFs activation and promotes laryngeal squamous cell carcinoma metastasis. Discov Oncol. 2022;13:120. doi: 10.1007/s12672-022-00578-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, Qiao P, Zhang J, Huang S, Hu A. Rho-associated kinase1 promotes laryngeal squamous cell carcinoma tumorigenesis and progression via the FAK signaling pathway. Discov Oncol. 2022;13:100. doi: 10.1007/s12672-022-00561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu YY, Ma CC, Ai KX. Knockdown of RAD51AP1 suppressed cell proliferation and invasion in esophageal squamous cell carcinoma. Discov Oncol. 2022;13:101. doi: 10.1007/s12672-022-00566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou WH, Wang Y, Yan C, Du WD, Al-Aroomi MA, Zheng L, et al. CC chemokine receptor 7 promotes macrophage recruitment and induces M2-polarization through CC chemokine ligand 19&21 in oral squamous cell carcinoma. Discov Oncol. 2022;13:67. doi: 10.1007/s12672-022-00533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genenger B, Perry JR, Ashford B, Ranson M. A tEMTing target? Clinical and experimental evidence for epithelial-mesenchymal transition in the progression of cutaneous squamous cell carcinoma (a scoping systematic review) Discov Oncol. 2022;13:42. doi: 10.1007/s12672-022-00510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–74. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Guan Y, Li C, Du H, Liang C. Identification and validation of a novel cuproptosis-related lncRNA gene signature to predict prognosis and immune response in bladder cancer. Discov Oncol. 2022;13:133. doi: 10.1007/s12672-022-00596-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Li L, Jiang X, Wang B, Hu X, Liu W, et al. Silencing of long non-coding RNA TUC338 inhibits the malignant phenotype of nasopharyngeal cancer cells via modulating the miR-1226-3p/FGF2 axis. Discov Oncol. 2022;13:102. doi: 10.1007/s12672-022-00544-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Ma Y, Di Y, Li Q, Zhan Q, He X, Liu S, et al. LncRNAs as epigenetic regulators of epithelial to mesenchymal transition in pancreatic cancer. Discov Oncol. 2022;13:61. doi: 10.1007/s12672-022-00522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei W, Xu T, Zhang Y, Huang Y, Wang X. Upregulation of long noncoding RNA linc02544 and its association with overall survival rate and the influence on cell proliferation and migration in lung squamous cell carcinoma. Discov Oncol. 2022;13:41. doi: 10.1007/s12672-022-00501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Zhuang Y, Fu X, Li C. LncRNA POU3F3 promotes melanoma cell proliferation by downregulating lncRNA MEG3. Discov Oncol. 2021;12:21. doi: 10.1007/s12672-021-00414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slack FJ, Chinnaiyan AM. The role of non-coding RNAs in oncology. Cell. 2019;179:1033–55. doi: 10.1016/j.cell.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panatta E, Lena AM, Mancini M, Smirnov A, Marini A, Delli Ponti R, et al. Long non‐coding RNA uc.291 controls epithelial differentiation by interfering with the ACTL6A/BAF complex. EMBO Rep. 2020;21:e46734. doi: 10.15252/embr.201846734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mancini M, Cappello A, Pecorari R, Lena AM, Montanaro M, Fania L, et al. Involvement of transcribed lncRNA uc.291 and SWI/SNF complex in cutaneous squamous cell carcinoma. Discov Oncol. 2021;12:14. doi: 10.1007/s12672-021-00409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Pajares V, Qu K, Zhang J, Webster DE, Barajas BC, Siprashvili Z, et al. A LncRNA-MAF:MAFB transcription factor network regulates epidermal differentiation. Dev Cell. 2015;32:693–706. doi: 10.1016/j.devcel.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soares E, Zhou H. Master regulatory role of p63 in epidermal development and disease. Cell Mol Life Sci. 2018;75:1179–90. doi: 10.1007/s00018-017-2701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pecorari R, Bernassola F, Melino G, Candi E. Distinct interactors define the p63 transcriptional signature in epithelial development or cancer. Biochem J. 2022;479:1375–92. doi: 10.1042/BCJ20210737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gatti V, Fierro C, Compagnone M, La Banca V, Mauriello A, Montanaro M, et al. DeltaNp63-Senataxin circuit controls keratinocyte differentiation by promoting the transcriptional termination of epidermal genes. Proc Natl Acad Sci USA. 2022;119:e2104718119. doi: 10.1073/pnas.2104718119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Straub WE, Weber TA, Schafer B, Candi E, Durst F, Ou HD, et al. The C-terminus of p63 contains multiple regulatory elements with different functions. Cell Death Dis. 2010;1:e5. doi: 10.1038/cddis.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lena AM, Rossi V, Osterburg S, Smirnov A, Osterburg C, Tuppi M, et al. The p63 C-terminus is essential for murine oocyte integrity. Nat Commun. 2021;12:383. doi: 10.1038/s41467-020-20669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin-Shiao E, Lan Y, Welzenbach J, Alexander KA, Zhang Z, Knapp M, et al. p63 establishes epithelial enhancers at critical craniofacial development genes. Sci Adv. 2019;5:eaaw0946. doi: 10.1126/sciadv.aaw0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu J, Yi G, Zhou H. p63 cooperates with CTCF to modulate chromatin architecture in skin keratinocytes. Epigenetics Chromatin. 2019;12:31. doi: 10.1186/s13072-019-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bao X, Rubin AJ, Qu K, Zhang J, Giresi PG, Chang HY, et al. A novel ATAC-seq approach reveals lineage-specific reinforcement of the open chromatin landscape via cooperation between BAF and p63. Genome Biol. 2015;16:284. doi: 10.1186/s13059-015-0840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu W, Guo Z, Liang P, Jiang B, Guo L, Duan M, et al. Expression changes in protein-coding genes and long non-coding RNAs in denatured dermis following thermal injury. Burns. 2020;46:1128–35. doi: 10.1016/j.burns.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Rubin AJ, Barajas BC, Furlan-Magaril M, Lopez-Pajares V, Mumbach MR, Howard I, et al. Lineage-specific dynamic and pre-established enhancer-promoter contacts cooperate in terminal differentiation. Nat Genet. 2017;49:1522–8. doi: 10.1038/ng.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donohue LKH, Guo MG, Zhao Y, Jung N, Bussat RT, Kim DS, et al. A cis-regulatory lexicon of DNA motif combinations mediating cell-type-specific gene regulation. Cell Genomics. 2022;2:100191. doi: 10.1016/j.xgen.2022.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KH, Son ED, Kim HJ, Lee SH, Bae IH, Lee TR. EGR3 is a late epidermal differentiation regulator that establishes the skin-specific gene network. J Invest Dermatol. 2019;139:615–25. doi: 10.1016/j.jid.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Boxer LD, Barajas B, Tao S, Zhang J, Khavari PA. ZNF750 interacts with KLF4 and RCOR1, KDM1A, and CTBP1/2 chromatin regulators to repress epidermal progenitor genes and induce differentiation genes. Genes Dev. 2014;28:2013–26. doi: 10.1101/gad.246579.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson B, Tilli CM, Hardman MJ, Avilion AA, MacLeod MC, Ashcroft GS, et al. Late cornified envelope family in differentiating epithelia—response to calcium and ultraviolet irradiation. J Invest Dermatol. 2005;124:1062–70. doi: 10.1111/j.0022-202X.2005.23699.x. [DOI] [PubMed] [Google Scholar]
- 38.Panatta E, Lena AM, Mancini M, Affinati M, Smirnov A, Annicchiarico-Petruzzelli M, et al. Kruppel-like factor 4 regulates keratinocyte senescence. Biochem. Biophys. Res Commun. 2018;499:389–95. doi: 10.1016/j.bbrc.2018.03.172. [DOI] [PubMed] [Google Scholar]
- 39.Butera A, Agostini M, Cassandri M, De Nicola F, Fanciulli M, D’Ambrosio L, et al. ZFP750 affects the cutaneous barrier through regulating lipid metabolism. Sci Adv. 2023;9:eadg5423. doi: 10.1126/sciadv.adg5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahlen P, Spalinskas R, Asad S, Mahapatra KD, Hojer P, Anil A, et al. Chromatin interactions in differentiating keratinocytes reveal novel atopic dermatitis- and psoriasis-associated genes. J Allergy Clin Immunol. 2021;147:1742–52. doi: 10.1016/j.jaci.2020.09.035. [DOI] [PubMed] [Google Scholar]
- 41.Sen GL, Boxer LD, Webster DE, Bussat RT, Qu K, Zarnegar BJ, et al. ZNF750 is a p63 target gene that induces KLF4 to drive terminal epidermal differentiation. Dev Cell. 2012;22:669–77. doi: 10.1016/j.devcel.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smirnov A, Lena AM, Cappello A, Panatta E, Anemona L, Bischetti S, et al. ZNF185 is a p63 target gene critical for epidermal differentiation and squamous cell carcinoma development. Oncogene. 2019;38:1625–38. doi: 10.1038/s41388-018-0509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiedemann J, Billi AC, Bocci F, Kashgari G, Xing E, Tsoi LC, et al. Differential cell composition and split epidermal differentiation in human palm, sole, and hip skin. Cell Rep. 2023;42:111994. doi: 10.1016/j.celrep.2023.111994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kouwenhoven EN, Oti M, Niehues H, van Heeringen SJ, Schalkwijk J, Stunnenberg HG, et al. Transcription factor p63 bookmarks and regulates dynamic enhancers during epidermal differentiation. EMBO Rep. 2015;16:863–78. doi: 10.15252/embr.201439941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo Y, Hitz BC, Gabdank I, Hilton JA, Kagda MS, Lam B, et al. New developments on the Encyclopedia of DNA Elements (ENCODE) data portal. Nucleic Acids Res. 2020;48:D882–9. doi: 10.1093/nar/gkz1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.GTEx-consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–5. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36:411–20. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A, et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol. 2019;20:163–72. doi: 10.1038/s41590-018-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lambert SA, Jolma A, Campitelli LF, Das PK, Yin Y, Albu M, et al. The human transcription factors. Cell. 2018;172:650–65. doi: 10.1016/j.cell.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 53.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4:1686. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the high-throughput sequencing data used in this study are publicly available and the accession numbers are listed in Materials and Methods.