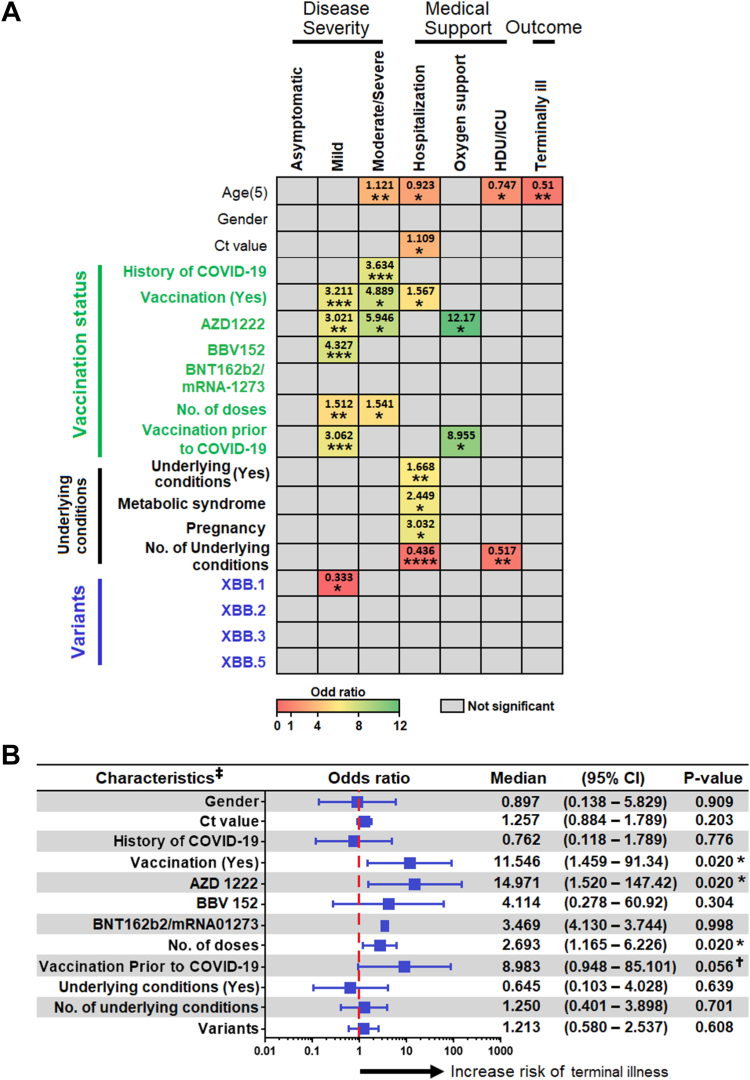
Fig. 2.
(A) Univariate binary regression analysis of factors associated with disease severity, use of medical supports and disease outcome. The odds ratio of a significant association is depicted according to the colour scale. Odds ratio of an insignificant association is depicted in grey. The complete regression analysis results were provided in Supplemental Table S7A–S7G. ∗, ∗∗, ∗∗∗, ∗∗∗∗ represent p < 0.05, <0.01, <0.001 and <0.0001, respectively. (B) Association of patients’ characteristics with risk of development of terminal illness. Given that the age was the only variable that associated with terminal illness in univariate analysis, here the regression analysis was performed controlling for age. Footnotes: ∗p < 0.05; †Having a trend of significant associated; ‡All variable were adjusted for age. Age (5) represents increase of every 5 years. Ct, cycle threshold; HDU, high dependency unit; ICU, intensive care unit. BNT162b2 (Pfizer-BioNTech) is a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine; mRNA-1273 is an mRNA-based vaccine that contains elasomeran, which instructs the host cell to produce a protein from the original strain of SARS-CoV-2; AZD1222 is an adenoviral vector-based vaccine; BBV152 is a whole-virion-based inactivated vaccine.