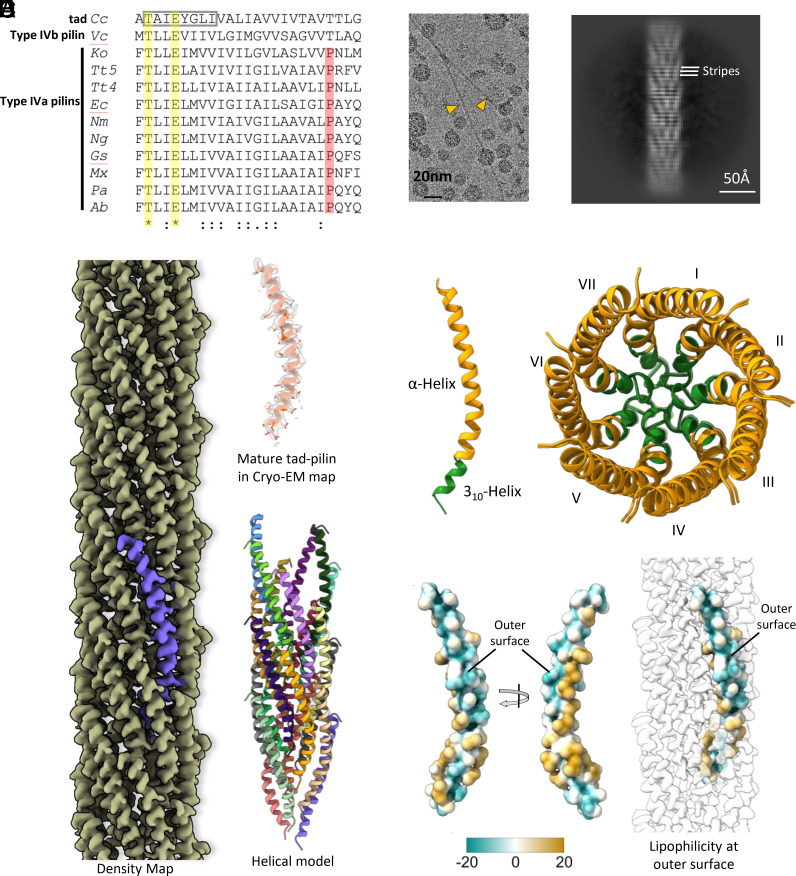
Fig. 1.
Cryo-EM structure and sequence analysis of the C. crescentus tad pilus. (A) Multiple sequence alignment of the N-terminal residues for all bacterial T4P and endopili whose structure has been determined. The abbreviations are: Cc, C. crescentus tad (PDB 8U1K); Vc, V. cholerae TCP (PDB 8U74); Ko, Klebsiella oxytoca PulG endopilin (PDB 5WDA); Tt5, Thermus thermophilus PilA5 (PDB 6XXE); Tt4, Thermus thermophilus PilA4 (PDB 6XXD); Ec, Enterohemorrhagic Escherichia coli (PDB 6GV9); Nm, Neisseria meningitidis (PDB 5KUA); Ng, N. gonorrhoeae (PDB 5VXX); Gs, Geobacter sulfurreducens (PDB 6VK9); Mx, Myxococcus xanthus (PDB 8TJ2); Pa, Pseudomonas aeruginosa PAK (PDB 5VXY); and Ab, Acinetobacter baumanii (PDB 8TOB). The symbols are (*) for completely conserved, (:) for strong conservation, and (.) for weak conservation. Sequence conservation is not evident beyond residue 25 and the alignment is truncated there. The characteristic 8-residue flp motif in the tad pilin is shown by the gray box. The only completely conserved residues in these structures are Thr2 and Glu5 (yellow highlighting). Aside from Thr2 and Glu5, all residues showing any degree of conservation are hydrophobic. Type IV pilins in all bacterial T4P structures published to date have a Pro22 (red highlighting), found in a partially melted region of the N-terminal helix. Both the C. crescentus tad pilus and V. cholerae TCP have a threonine at this position, and their helices are continuous. (B) Cryo-EM image showing two tad pili. (C) 2D class average, with horizontal stripes with a periodicity of 4.9 Å indicated. (D) Cryo-EM density map and structure of the C. crescentus tad pilus. (E) Secondary structure of Tad pilin (Left) and top view of the tad pilus in ribbon representation showing the 7-start helical organization. The flp motif forming a 310 helix is colored green, participating in the core interactions of the filaments. (F) Surface lipophilicity of tad pilin showing the polar patch on the C-terminus forming the filament outer surface. Polar and hydrophobic surfaces are represented by green and gold, respectively.