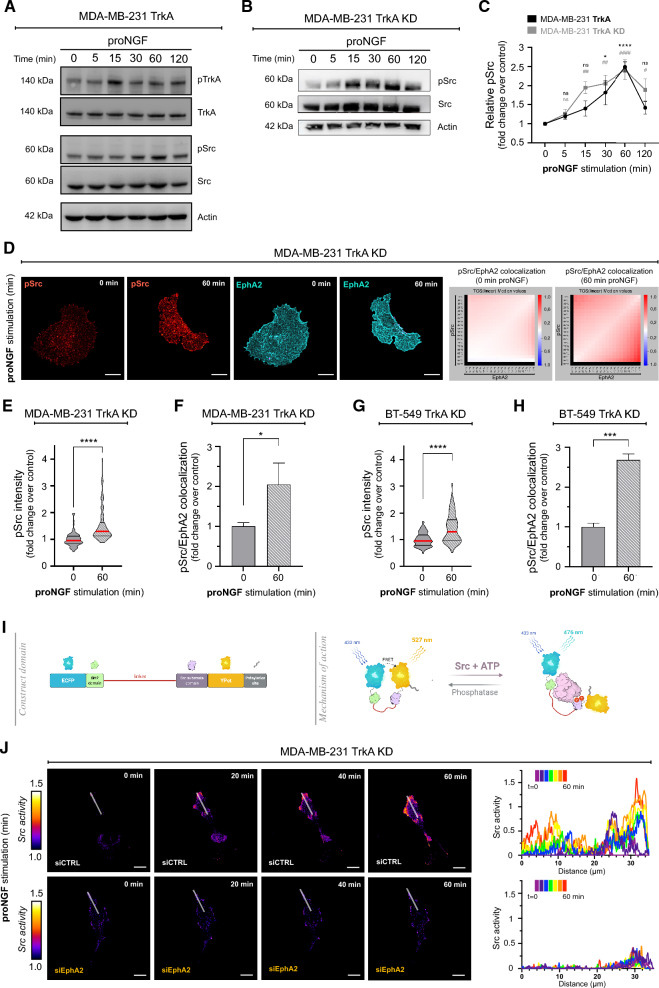
Fig. 2.
proNGF induces Src activation independently of TrkA phosphorylation in TNBC. A–C Immunoblotting from MDA-MB-231-TrkA (A) or MDA-MB-231-TrkA-KD (B) following proNGF treatment (5, 15, 30,60 and 120 min) for TrkA, Src (and their phosphorylation form) and actin with associated relative quantification of Src phosphorylated regarding to its unphosphorylated form (C). D-H Representative confocal images from proNGF simulated (0 or 60 min) MDA-MB-231-TrkA-KD (D) of Src active form (Red) and EphA2 (Blue) staining with pSrc/EphA2 colocalization matrix and respective Src activity (E & F) and EphA2/pSrc colocalization quantification (G & H) for MDA-MB-231-TrkA-KD and BT-549 KD. I Scheme of FRET Src reporter from Ouyang et al. that is characterized by an ECFP/YPET pair, an SH2 domain, a Src substrate domain and a prenylation site. (J) Emission ratio images and profiles along the indicated white lines of the ECFP/YPet-based Src biosensor in response to proNGF stimulation with or without prior transfection of siEphA2 on MDA-MB-231-TrkA-KD. Excluding experiment B which is performed in duplicate, data in A to J are representative of 3 independents experiments. For D to H, they are performed on 30 cells/condition each, data in J are realized on approximately 7 cells/condition. Data in C are represented by connecting line graph with mean ± SEM. Data in E and G are presented with violin plots demonstrating the median (red bold line), quartiles (thin black line), variability and density. Data in F and H are represented by column bar graph with SD. Two-way ANOVA followed by Sidak’s test for C with the set of conditions was compared to the corresponding 0 min time. Unpaired 2-tailed t test for E to H. * and #P ≤ 0.05, ** and ##P ≤ 0.01, *** and ###P ≤ 0.001, **** and ####P ≤ 0.0001. For D and E, scale bare = 15 µm