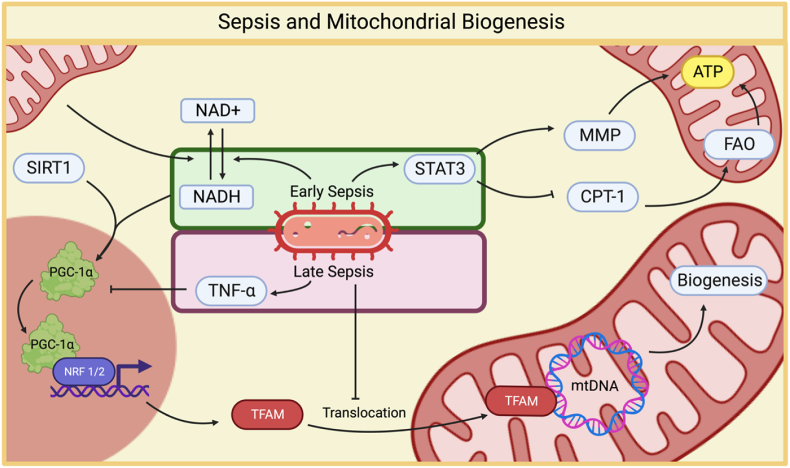
Fig. 2.
The relationship between sepsis and mitochondrial biogenesis. During the initial stages of sepsis, there is a notable downregulation of mitochondrial biogenesis. This reduction is primarily driven by elevated levels of the cytokine TNFα, which leads to decreased levels of PGC-1α [86]. As sepsis progresses to its later stages, mitochondrial dysfunction becomes increasingly evident. In response to this dysfunction, a defensive cellular response is initiated to counteract oxidative stress and prevent cell death. STAT3 enhances the mitochondrial membrane potential, thus improving ATP production efficiency, which is crucial for cellular energy supply. In an effort to maintain energy production, cells increase fatty acid oxidation. This is achieved, in part, by inhibiting the degradation of CPT1, a key enzyme involved in fatty acid transport into mitochondria [87] Additionally, the increased expression of Nrf1 and Nrf2, along with TFAM, suggests an upregulated response to cope with dysfunctional mitochondria [2]. However, a notable challenge emerges in the form of impaired TFAM translocation into the mitochondria [88]. This impairment potentially explains the contradictory results observed regarding mitochondrial biogenesis in sepsis.