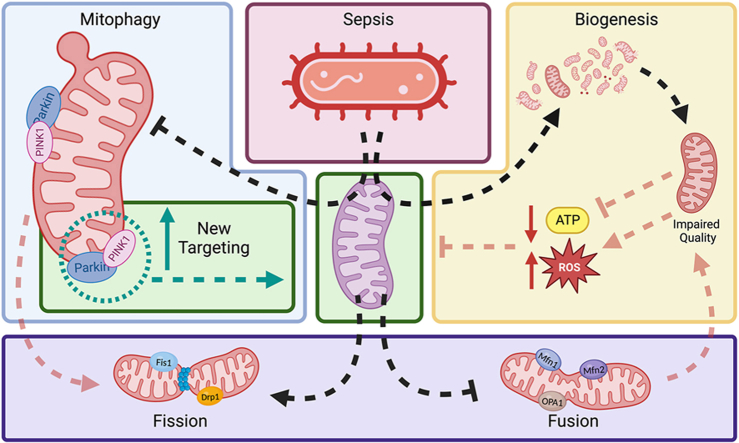
Under healthy conditions (depicted in purple), mitochondria maintain a delicate balance of mtQC mechanisms to ensure optimal mitochondrial mass and function. However, in sepsis (highlighted in the red block), multiple factors disrupt this equilibrium. As sepsis progresses, mitochondrial dysfunction becomes evident, characterized by reduced ATP production and increased ROS [1]. This dysfunctional state triggers a series of responses aimed at mitigating damage and restoring mitochondrial health. Excessive mitochondrial proliferation (depicted in the yellow block) occurs as a compensatory mechanism, attempting to counteract the deteriorating mitochondrial function [2]. Simultaneously, mitophagy is inhibited (depicted in the blue block), further contributing to mitochondrial dysfunction [3]. Additionally, increased mitochondrial fission (highlighted in the purple block) is observed as a response to sepsis-induced stress [4]. To restore cellular homeostasis, it is crucial to promote patent mitophagy and shift the balance towards fusion, allowing for the removal of faulty mitochondria. Only after successful induction of mitophagy can upregulation of mitochondrial biogenesis be beneficial for restoring mitochondrial mass and bioenergetics. This graphical abstract highlights the intricate interplay between mtQC mechanisms in sepsis, emphasizing the importance of restoring mitochondrial health to maintain overall cellular homeostasis.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.