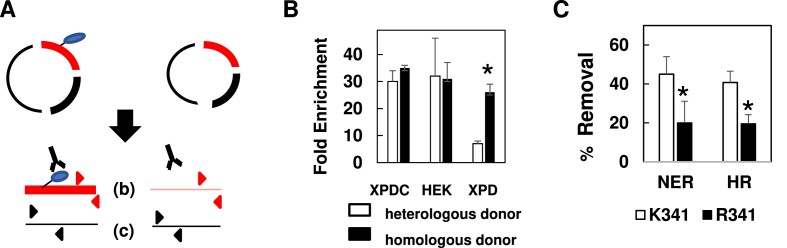
Figure 3.
DPCs are ubiquitinated in NER and HR-mediated repair. (A) Schematic of anti-ubiquitin IP-qPCR. Anti-ubiquitin antibody is used to specifically enrich for ubiquitin (and any covalently bound protein or DNA). qPCR is then used to quantify the abundance of the DPC-containing fragment (red) relative to the control, non DPC-containing fragment (black). See text for details. (B) DPCs were transfected into cells proficient for NER (XPDC, HEK) or deficient for NER (XPD) in the presence of a heterologous or homologous donor plasmid. Low molecular weight DNA was recovered from cells one-h post transfection and subjected to anti-ubiquitin IP-qPCR. Results depict fold-enrichment of DPC containing fragment following antibody treatment, measured by qPCR, *P= 0.02. (C) DPC substrates produced by cross-linking the wild-type OGG1 protein (K341) or arginine for lysine-substituted version (R341). DPCs were transfected into HEK293T cells in the absence of homologous donor into NER proficient cells (NER) or in the presence of homologous donor into NER deficient cells (HR) cells. Low molecular weight DNA was recovered 1 h following transfection. DPCs were then subjected to KCl-SDS-qPCR, as described above, to determine the percentage of crosslinked protein removed (% Removal), *P= 0.05.