Figure 2. A conserved hydrophilic reaction chamber within Lac1.
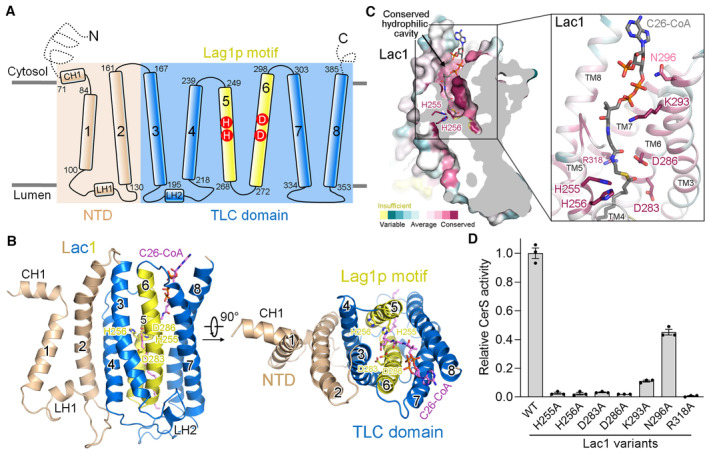
- Schematic representation of the topology of Lac1. NTD, N‐terminal domain; TLC, TRAM‐LAG1‐CLN8; CH, cytosolic helix; LH, luminal helix.
- Two perpendicular views of the Lac1 subunit. The NTD is colored wheat. The TLC domain is colored marine with the Lag1p motif shown in yellow. C26‐CoA is shown in magenta sticks. The predicted catalytic histidine and aspartate residues in the Lag1p motif are shown in sticks.
- A conserved hydrophilic cavity within the TLC domain of Lac1. Lac1 is colored by the amino acid conservation scores calculated by ConSurf (Yariv et al, 2023) analysis of the yeast and human CerS members presented in Fig EV1. The conserved charged and polar residues within the hydrophilic cavity are shown in sticks. C26‐CoA is shown in gray sticks.
- Functional characterization of the conserved charged and polar residues shown in panel (C) by CerS activity. The activities of Lac1‐Lip1 variants were normalized relative to that of the WT Lac1‐Lip1 complex. Each data point is the average ± SEM of three independent experiments.
Source data are available online for this figure.
