Figure 3. The acyl chain binding tunnel.
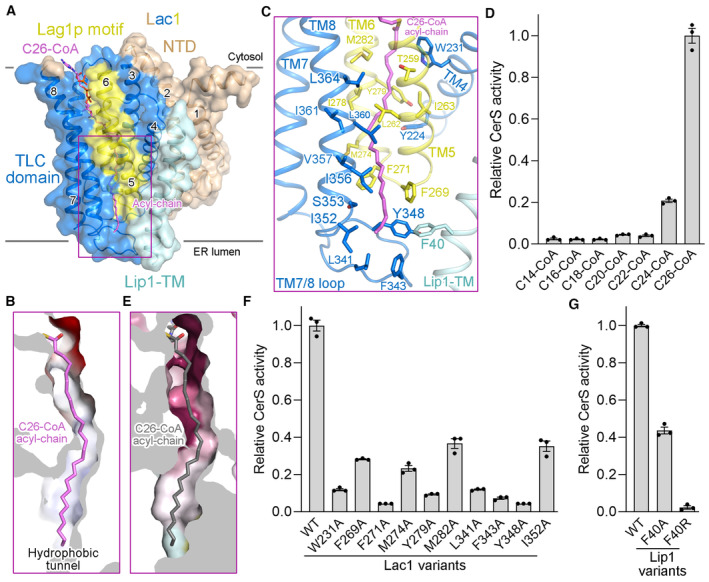
-
AC26‐CoA is coordinated by the TLC domain of Lac1 and the TM of Lip1.
-
BA hydrophobic tunnel for C26‐CoA acyl‐chain binding in Lac1.
-
CA close‐up view of the interactions between C26‐CoA acyl‐chain and the Lac1‐Lip1 complex. The residues lining the acyl chain binding tunnel are shown in sticks.
-
DAcyl‐chain selectivity of the Lac1‐Lip1 complex revealed by CerS activity. Each data point is the average ± SEM of three independent experiments.
-
EThe distal end of the hydrophobic tunnel for C26‐CoA acyl‐chain coordination is not conserved. Lac1 is colored by the same amino acid conservation scores as in Fig 2C.
-
F, GFunctional characterization of Lac1 (F) and Lip1 (G) hydrophobic residues for C26‐CoA acyl‐chain binding by CerS activity. Each data point is the average ± SEM of three independent experiments.
Source data are available online for this figure.
