Figure EV4. Proposed working mechanism of yeast CerS.
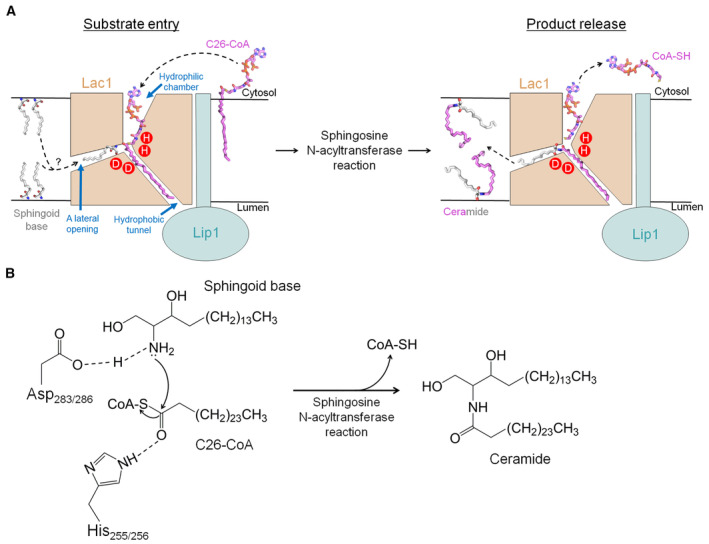
- A hypothetic model for yeast CerS‐catalyzed ceramide formation. The C26‐CoA substrate enters the reaction chamber within Lac1 probably through the cytosolic side of the membrane. The CoA moiety of C26‐CoA is located in the hydrophilic reaction chamber, while the acyl‐chain moiety is positioned in the hydrophobic tunnel. Once the sphingoid base substrate from either leaflet of the membrane enters a lateral opening on Lac1, it approaches the catalytic histidine and aspartate residues near the thioester bond of C26‐CoA, preparing for the sphingosine N‐acyltransferase reaction. After the reaction, the product of free CoA‐SH is released to the cytosol, whereas the hydrophobic product ceramide is released to either leaflet of the membrane. For simplicity, only one Lac1‐Lip1 heterodimer is illustrated.
- Proposed catalytic mechanism. Asp283 or Asp286 of Lac1 functions as a general base to activate the amino group of the sphingoid base for nucleophilic attack on the carbonyl carbon of C26‐CoA, which is coordinated by His255 or His256. The CerS‐catalyzed acyl transfer reaction promotes the N‐acylation of sphingoid base for ceramide production and releases free CoA‐SH.
