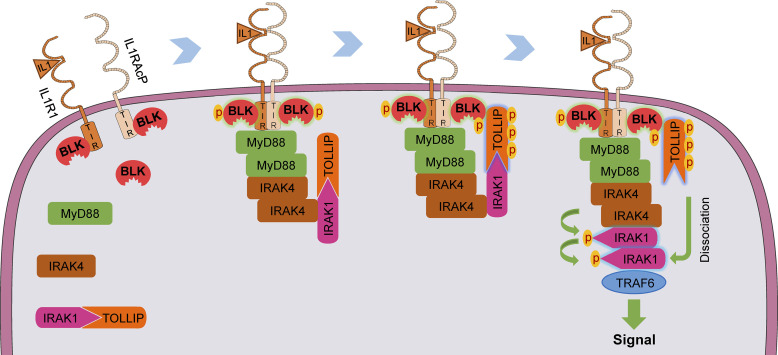
Figure 9.
A schematic presentation for the role of BLK in regulating TLR/IL-1R–mediated inflammatory response. Under unstimulated conditions, BLK is constitutively associated with the cytoplasmic TIR domains of IL1R1 and IL1RAcP. Upon binding of IL-1β to IL1R1, IL1RAcP is recruited to form a high-affinity heterodimer, which results in the conformational change of TIR domains and further triggers BLK autophosphorylation. Simultaneously, the receptor complex recruits MyD88, IRAKs, and TOLLIP to form a signal transduction complex termed Myddosome. Activated BLK phosphorylates TOLLIP and further promotes TOLLIP dissociation from IRAK1 by competitively binding to the CUE domain of TOLLIP, thus enabling downstream signaling cascade.