Abstract
Context:
Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) may adversely affect bone by inducing oxidative stress. Whether this translates into increased fracture risk in older adults is uncertain.
Objective:
Determine the associations of plasma TMAO with hip fracture and bone mineral density (BMD) in older adults
Design and Setting:
Cox hazard models and linear regression stratified by sex examined the associations of TMAO with hip fracture and BMD in the longitudinal cohort of the Cardiovascular Health Study
Participants:
5019 U.S. adults aged ≥65 years
Exposure:
Plasma TMAO
Main Outcome Measures:
Incident hip fractures; total hip BMD dual x-ray absorptiometry in a subset (n=1400)
Results:
Six hundred sixty-six incident hip fractures occurred during up to 26 years of follow-up (67,574 person-years). After multivariable adjustment, TMAO was not significantly associated with hip fracture (women: hazard ratio (HR) [95% confidence interval (CI)] of 1.00[0.92,1.09] per TMAO doubling; men: 1.12[0.95,1.33]). TMAO was also not associated with total hip BMD (women: BMD difference [95% CI] of 0.42 g/cm2*100 [−0.34,1.17] per TMAO doubling; men: 0.19[−1.04,1.42]). In exploratory analyses, we found an interaction between body mass index (BMI) and the association of TMAO with hip fracture (P<0.01). Higher TMAO was significantly associated with risk of hip fracture in adults with overweight or obesity (BMI≥25) (HR [95% CI]:1.17[1.05,1.31]), but not normal or underweight.
Conclusions:
Among older US men and women, TMAO was not significantly associated with risk of hip fracture or BMD overall. Exploratory analyses suggested a significant association between higher TMAO and hip fracture when BMI was elevated, which merits further study.
Keywords: trimethylamine N-oxide, gut microbiome, bone mineral density, hip fracture, osteoporosis, animal food
1. Introduction
The gut microbiome is important for bone health (1,2). In mice, probiotic treatment has been reported to change gut microbiome composition and mitigate age-related bone loss (3,4). Multiple randomized control trials in postmenopausal women have shown probiotics reduce loss of bone mineral density (BMD) (5–7). Trimethylamine N-oxide (TMAO) is a metabolite measurable in plasma and produced when dietary L-carnitine, a nutrient abundant in red meat, and phosphatidylcholine, a nutrient in eggs, cheese, meats and seafood, are converted to trimethylamine by the gut microbiome and oxidized to TMAO in the liver by flavin-containing monooxygenase 3 (FMO3) (8,9). TMAO is renally cleared (>95% clearance in healthy individuals) (10). In animal models, TMAO has been shown to independently cause renal damage and elevated cystatin C (11). Plasma TMAO levels are further impacted by gut microbiome composition (12) and increase with age (13–15). There are several lines of evidence suggesting TMAO may be associated with bone health, but its association with clinical measures of bone health in humans, such as low BMD and fracture, is uncertain.
Lower plasma levels of antioxidants were associated with osteoporosis in postmenopausal women, and higher levels of reactive oxygen species (ROS) were associated with lower BMD in adults, suggesting that higher oxidative stress contributes to osteoporosis development (16,17). One proposed mechanism is that oxidative stress facilitates osteoclastogenesis through nuclear factor κB (NF-κB) activation (18), leading to inflammation via various pathways including triggering the nucleotide-binding oligomerization domain-like (NOD-like) receptor protein 3 (NLRP3) inflammasome (19). In experimental models, TMAO induces oxidative stress (20,21). TMAO may also promote adipogenic and inhibit osteogenic differentiation of bone marrow mesenchymal stem cells through increased oxidative stress and NF-κB activation (22). In aging mice, a traditional Chinese medicine modulated gut microbiome composition, decreased hepatic FMO3 expression, decreased plasma TMAO, reduced NF-κB signaling, and promoted bone quality, suggesting TMAO may exert modifiable pro-osteoporotic effects by inducing oxidative stress (23).
In small cross-sectional or retrospective case-control studies of older people, higher TMAO was associated with low BMD (n=10 cases) (22) and hip fracture (n=286 cases) (24). In contrast, a small longitudinal study (n=264), conducted as a post-hoc analysis of a weight loss trial, showed decreases in TMAO over time were associated with greater loss in spine and whole-body BMD in persons with overweight and obesity (25). These prior studies, limited in scope by their size and design, provide uncertain and conflicting evidence on the relationship between TMAO and osteoporosis. Whether TMAO is associated with clinical metrics of bone health in older adults remains unclear. To address these gaps in knowledge, the present study examined the hypothesis that higher TMAO is cross-sectionally associated with lower total hip BMD and prospectively associated with higher hip fracture incidence among more than 5,000 U.S. community-dwelling adults aged ≥65 years with long-term follow-up data.
2. Participants and Methods
2.1. Study participants
The Cardiovascular Health Study (CHS) is a longitudinal study of community-dwelling adults aged 65 years and older from four U.S. sites drawn from Medicare eligibility lists (26). The original cohort of 5,201 participants was enrolled in 1989–1990, and an additional 687 African Americans were enrolled in 1992–1993. Institutional review boards approved the study at all sites and all participants provided written informed consent. From 1989 to 1999, participants underwent annual clinic examinations including blood samples. From 1999 to June 30, 2015, participants were surveyed biennially for incident hospitalizations, diagnoses, and medications.
2.2. Analytic cohorts
Eligible participants were men and women with stored serum available at baseline for TMAO measurement (1989–1990 or 1992–1993 in the original and African American cohorts) (n=5,019) (baseline TMAO-incident hip fracture cohort). Compared with CHS participants without TMAO measured, this analytic cohort was younger, had a higher percentage of women, African Americans, and participants with prevalent myocardial infarction and a lower percentage of participants with prevalent diabetes (Supplementary Table 1). Two of the four CHS sites performed dual-energy x-ray absorptiometry (DXA) during 1994–1995 on 1,591 CHS participants. Of those individuals, 1,400 also had baseline stored serum for TMAO measurement (BMD cohort). TMAO was measured again at the 1996–1997 visit in a subset (n=2,995) of participants with baseline TMAO. These individuals were included in a pre-specified secondary analysis to determine if change in TMAO concentrations over time were associated with hip fracture risk (change in TMAO-incident hip fracture cohort) (Figure 1).
Figure 1.
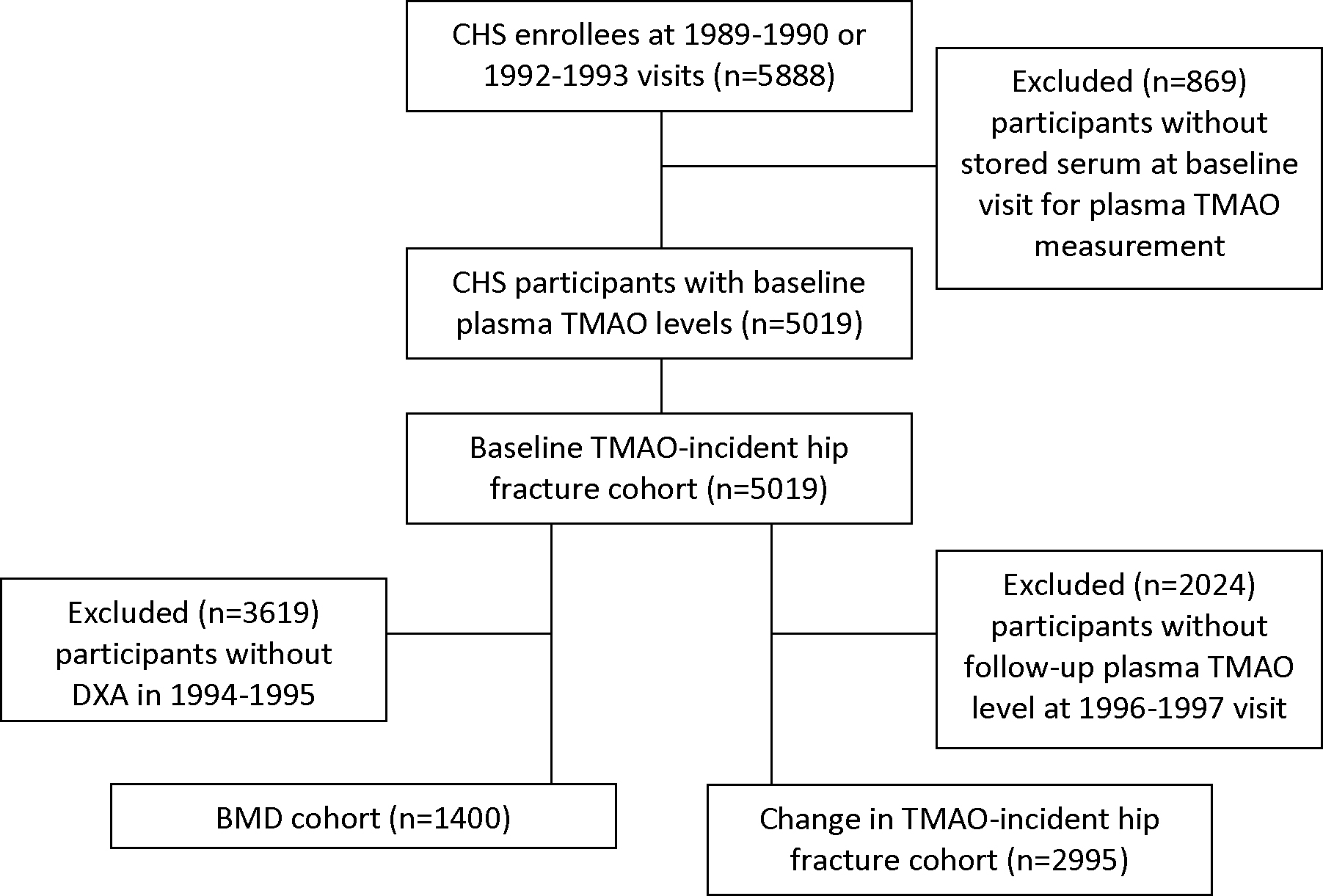
Analytic cohorts
CHS: Cardiovascular Health Study; TMAO: Trimethylamine N-oxide; DXA: Dual X-ray absorptiometry; BMD: bone mineral density
2.3. Quantification of trimethylamine N-oxide
Blood samples were collected after a 12-hour fast and stored at −80 °C at the University of Vermont, in Colchester, VT. Stable-isotope-dilution liquid chromatography with tandem mass spectrometry (LC-MS/MS) was used for quantification of TMAO plasma. Ice-cold methanol solution of internal standard (d9-TMAO) was added to plasma samples, followed by vortexing and centrifuging (21,000 × g; 4 °C for 15 min). The clear supernatant was then transferred to glass vials with microinserts. LC-MS/MS analysis was performed on a chromatographic system consisting of two Shimadzu LC-30 AD pumps (Nexera X2), a CTO 20AC oven operating at 30 °C, and a SIL-30 AC-MP autosampler in tandem with 8050 triple quadruple mass spectrometer (Shimadzu Scientific Instruments, Inc., Columbia, MD, USA). Electrospray ionization in positive ion mode with multiple reaction monitoring (MRM) was used with the following parent → daughter ion transitions monitored: m/z 76 → 59 for TMAO and 85 → 66 for d9-TMAO (15). Intra-day and inter-day coefficients of variance were < 6.4% and < 9.9%, respectively (15). Stability studies showed that TMAO in plasma is stable both during storage at −80 °C for 5 years and to multiple freeze thaw cycles (15).
2.4. Assessment of outcomes
Incident hip fractures were identified by participant (or proxy) report every 6 months and then confirmed by hospital medical record review. In addition, hospital claims data and hospitalization discharge summaries were reviewed to capture fractures not reported by participants (27). Hip fracture was defined using the International Classification of Diseases, Ninth Revision (ICD-9), codes (820.xx). Pathological fractures (ICD-9 code 773.1x) and motor vehicle accidents (E810.xx-E825.xx) were excluded.
Total hip BMD was measured using Hologic QDR-2000 densitometers (Hologic, Inc., Waltham, MA, USA) and read at the University of California, San Francisco, Reading Center using Hologic software, version 7.10, by readers unaware of the participant’s TMAO measurements or other characteristics. All scans were completed using array beam mode and manufacturer’s recommended protocol dictated standardized positioning and use of QDR software. The coefficient of variation was <0.75% for all total hip BMD in CHS (28).
2.5. Assessment of covariates
Multivariate analyses were adjusted for factors measured at baseline that could impact TMAO and/or osteoporosis risk. These included age, sex, race, highest education level (≥12th or < 12th grade), study site, smoking history (status: current, former, never and total pack-years), current alcohol use (0 drinks/week, ≤7 drinks/week, >7 drinks/week), weight, height, body mass index (BMI; kg/m2), prevalent diabetes (26), prevalent cardiovascular disease (CVD; any of myocardial infarction, angina, congestive heart failure, stroke, transient ischemic attack or claudication) (29), estimated glomerular filtration rate (eGFR) from combined creatinine-cystatin C equation (30), high-sensitivity C reactive protein (CRP, log-transformed) (31), limitation in activities of daily living (ADLs; present if difficulty with one or more of the following: walking around the home, getting out of bed, eating, dressing, bathing, using the toilet) or instrumental ADLs (iADLs; present if difficulty with one or more of the following: heavy housework, light housework, shopping, preparing meals, paying bills, using the telephone), physical activity (kcal/week) (32), and frailty (frail, pre-frail, not frail) (33). Medications were determined via direct examination of medication bottles including osteoporosis medications (vitamin D, calcium, selective estrogen receptor modulators, estrogens, and bisphosphonates), oral corticosteroids, and other medications affecting bone metabolism and/or fracture risk (loop diuretics, thiazide diuretics, selective serotonin reuptake inhibitors, anticonvulsants, benzodiazepines, sedative/hypnotics, proton pump inhibitors, thiazolidinediones, and thyroid medications). Information on total animal source foods (servings/day of unprocessed red meat, processed meat, fish, chicken, or eggs) was obtained via administration of a validated picture-sort food frequency questionnaire in the original cohort at baseline (1989–1990). In the African American cohort, baseline (1992–1993) total animal source foods were imputed using demographic variables, time-varying lifestyle variables, medications, and other dietary variables based on data from the original cohort at baseline and food frequency questionnaire data from participants at the 1995–1996 study visit.
2.6. Statistical analyses
Because of sex differences in the natural history of hip fractures (34,35), we specified a priori that primary analyses were stratified by sex, but combined analyses were also performed. Baseline characteristics were described, stratified by sex and across quartiles of TMAO, for the baseline TMAO-incident hip fracture analytic cohort. Incidence rates of hip fracture, total and across quartiles of TMAO, were calculated with quasi-Poisson models with offset to accommodate time at risk. Kaplan-Meier plots displayed hip fracture-free survival by TMAO quartiles. A log-rank test across TMAO quartiles was performed.
In multivariate analyses, continuous data on TMAO was log2-transformed (hereafter called log2TMAO). Multivariable Cox hazards models were used to estimate the hazard ratios (HR) and 95% confidence intervals (CI) of incident hip fracture associated with log2TMAO. Follow-up for incident hip fractures began at the time of baseline TMAO measurement and was continued until hip fracture event, death, last contact in those without data from CMS, or June 30, 2015, whichever occurred first. To minimize confounding, we used nested models adjusting for factors as: Model 0 (M0) = unadjusted; Model 1 (M1) = age, race, sex, study site, and education level; Model 2 (M2) = M1 + smoking status, smoking total pack-years, alcohol use, prevalent diabetes, prevalent cardiovascular disease, BMI, physical activity, limitation in ADLs, limitation in iADLs, frailty, osteoporosis medication use, oral corticosteroid use, and use of other medications affecting bone metabolism and/or fracture risk; Model 3 (M3) (the final multivariable adjusted model) = M2 + CRP and total animal source foods; and Model 4 (M4) (exploratory model) = M3 + eGFR, which in experimental models is causally influenced by TMAO (11) and thus could be either a mediator or confounder on the pathway between TMAO and osteoporosis development. We pre-specified to explore in M3 whether the relationship of TMAO and hip fracture differs by total animal source foods dietary intake (high or low, if above or below the median value).
In the BMD cohort, multivariable linear regression estimated the association between log2TMAO and total hip BMD using the same nested multivariable models as above and with M3 specified to be the final multivariable adjusted model.
Because obesity is characterized by oxidative stress and inflammation (36), persons with overweight or obesity may be particularly vulnerable to the hypothesized pro-inflammatory effects of TMAO on bone. Therefore, we queried whether BMI category was a potential effect modifier on the association of TMAO and hip fracture. We explored this in a post-hoc analysis. A statistically significant interaction with BMI was seen in the final multivariable adjusted model for the association of TMAO and hip fracture (P<0.01 overall; P<0.01 in women; P=0.13 in men). We estimated the association of log2TMAO with incident hip fracture and total hip BMD, stratified by BMI category: (i) BMI < 25 kg/m2 (normal weight or underweight), (ii) BMI ≥ 25 kg/m2 (overweight or obese), and (iii) BMI ≥ 30 kg/m2 (obese).
When assessing hip fracture risk associated with change in TMAO over time, scatter plots graphed TMAO and log2TMAO between baseline and follow-up (1996–1997) visits. Summary statistics on annualized change in TMAO were calculated, and the association of baseline with follow-up log2TMAO was determined using a Pearson’s correlation coefficient. Multivariable Cox hazards models were used to estimate the HR of incident hip fracture associated with the annualized change in log2TMAO, with follow-up for incident hip fracture beginning at the time of the second TMAO measurement (1996–1997). We used the same nested multivariable models, additionally adjusting for the baseline log2TMAO, with M3 specified to be the final multivariable adjusted model.
To maximize use of the available data on serial TMAO levels, a sensitivity analysis was conducted adding at the analysis baseline those with only a follow-up TMAO measure (n=399). Thus, at the analysis baseline, we combined the 5,019 participants with TMAO measure at year 1989–1990 (the original cohort) or 1992–1993 (the African American cohort) with 399 participants at year 1996–1997 who were missing a TMAO measure at their baseline time point. Follow-up for incident hip fracture began at the analysis baseline. In this sensitivity analysis, for individuals with more than one serial TMAO measure available before the incident hip fracture event (baseline and 1996–1997), we updated the exposure with this measure; that is, in the original cohort we updated after 7 years, and in the African American cohort after 4 years of follow-up (n=2,931). For those 399 participants with only a follow-up TMAO measure in 1996–1997 and those participants with baseline TMAO measure but missing a follow-up TMAO measure in 1996–1997, we did not update. Note that this approach has potentially several sources of bias: due to differential updating across cohorts that differ in race distribution; due to possible informative missingness of the TMAO updating measure; and due to non-representative analysis population. We used robust standard error estimates in this Cox regression, with participant as a cluster.
Because sarcopenia is an established non-skeletal determinant of fragility fracture (37,38) and is associated with increased fall risk (39), we queried whether measurements of muscle function (i.e. grip strength) and fall frequency were mediators between TMAO and hip fracture risk. We performed additional sensitivity analyses on the baseline TMAO-incident hip fracture cohort to explore this. We included (i) grip strength (kilograms) and, in a separate model, (ii) self-reported history of falls in the prior 6 months, overall and stratified by BMI category (BMI<25 kg/m2 versus BMI≥25 kg/m2), in M3 (the final multivariable adjusted model). Both grip strength and self-reported history of falls were ascertained at baseline. Because weight loss is associated with increased hip fracture risk (40,41), a final sensitivity analysis on the baseline TMAO-incident hip fracture cohort included weight as a time-varying covariate (measured annually through the 1998–1999 study visit, if still at risk) in M3.
Analyses were performed using R environment for statistical computing (42), with two-tailed alpha=0.05.
3. Results
3.1. Baseline characteristics
At baseline, the mean (standard deviation (SD)) age was 72.5 (5.4) with 38.3% men and 16.1% of participants identifying as African American (Table 1). The median [intraquartile range] baseline TMAO was 4.84 [3.26–7.91] μmol/L (range:0.01–254.99 μmol/L). Baseline characteristics by TMAO quartiles are shown in Supplementary Tables 2 and 3. In these crude (unadjusted) comparisons, TMAO varied by study site, and higher TMAO was associated with older age, higher CRP, lower eGFR and higher prevalence of diabetes and CVD in both men and women. In women, higher TMAO was associated with more smoking and more frequent limitations in ADLs and iADLs. Frailty and BMI category significantly varied across TMAO quartiles in women. In men, higher TMAO was associated with higher total animal source food intake, greater use of medications affecting bone metabolism and/or fracture risk, and lower percentage of men identifying as African American.
Table 1.
Characteristics of the Analytic Cohort at Baselinea
| Overall (n=5,019) | Women (n=3,099) | Men (n=1,920) | |
|---|---|---|---|
| TMAO - µmol/L | 4.84 [3.26–7.91] | 4.63 [3.11–7.55] | 5.25 [3.57–8.44] |
| Age - years | 72.5 ± 5.4 | 72.3 ± 5.4 | 72.8 ± 5.5 |
| African American Raceb – n (%) | 809 (16.1) | 525 (16.9) | 284 (14.8) |
| Study site – n (%) | |||
| Winston-Salem, NC | 1311 (26.1) | 830 (26.8) | 480 (25.0) |
| Hagerstown, MD | 1101 (21.9) | 814 (26.3) | 492 (25.6) |
| Sacramento, CA | 1305 (26.0) | 674 (21.7) | 427 (22.2) |
| Pittsburg, PA | 1302 (25.9) | 781 (25.2) | 521 (27.1) |
| Education (completed ≥ 12 years) – n (%) | 2151 (43.0) | 1238 (40.1) | 913 (47.8) |
| Smoking status – n (%) | |||
| Current | 612 (12.2) | 394 (12.7) | 218 (11.4) |
| Former | 2049 (40.9) | 949 (30.7) | 1100 (57.4) |
| Never | 2353 (46.9) | 1753 (56.6) | 600 (31.3) |
| Smoking – pack-years | 17.4 ± 26.5 | 12.1 ± 21.3 | 26.1 ± 31.4 |
| Alcohol use – n (%) | |||
| 0 drinks/week | 2518 (50.4) | 1728 (55.9) | 790 (41.3) |
| ≤7 drinks/week | 1875 (37.5) | 1095 (35.4) | 780 (40.8) |
| >7 drinks/week | 607 (12.1) | 266 (8.6) | 341 (17.8) |
| Prevalent diabetes – n (%) | 790 (15.8) | 441 (14.3) | 349 (18.2) |
| Prevalent CVDc – n (%) | 994 (19.8) | 488 (15.7) | 506 (26.4) |
| BMId - kg/m2 | 26.8 ± 4.8 | 26.9 ± 5.3 | 26.5 ± 3.8 |
| BMI categorye – n (%) | |||
| Normal or underweight | 1923 (38.4) | 1247 (40.3) | 676 (35.3) |
| Overweight | 2063 (41.2) | 1122 (36.3) | 941 (49.2) |
| Obese | 1019 (20.4) | 722 (23.4) | 297 (15.5) |
| Physical activity – kcal/week | 585 [105–1470] | 412.5 [19.4–1125] | 945 [315–2238.8] |
| Limitation in ADLsf – n (%) | 411 (8.2) | 299 (9.7) | 112 (5.9) |
| Limitation in iADLsg – n (%) | 1288 (25.7) | 932 (30.1) | 356 (18.6) |
| Frailtyh – n (%) | |||
| Not frail | 2121 (47.3) | 1292 (46.4) | 829 (48.7) |
| Prefrail | 2071 (46.1) | 1276 (45.8) | 795 (46.7) |
| Frail | 296 (6.6) | 219 (7.9) | 77 (4.5) |
| Corticosteroid usei – n (%) | 100 (2.0) | 59 (1.9) | 41 (2.1) |
| Osteoporosis medication usej – n (%) | 125 (2.5) | 120 (3.9) | 5 (0.3) |
| Other bone medication usek – n (%) | 1663 (33.1) | 1207 (39) | 456 (23.8) |
| C-reactive protein – mg/L | 2.6 ± 2.8 | 2.7 ± 2.8 | 2.4 ± 2.8 |
| Total animal source foodsl – servings/day | 1.7 ± 1.0 | 1.6 ± 0.9 | 1.9 ± 1.0 |
| eGFRm | 68.6 (17.1) | 69.2 (17.0) | 67.8 (17.3) |
TMAO: Trimethylamine N-oxide; NC: North Carolina; MD: Maryland; CA: California; PA: Pennsylvania; CVD: Cardiovascular disease
Entries show number (percentage) for binary and categorical variables and mean ± SD for continuous variables, except for TMAO and physical activity, which are reported as median [interquartile range]
Remainder of participants are almost exclusively white/Caucasian
Prevalent CVD is a composite of coronary heart disease, angina, heart failure, stroke, transient ischemic attack, and claudication
Body mass index defined as (mass in kilograms)/(height in meters)2
Normal weight or underweight defined as BMI < 25 kg/m2; Overweight defined as BMI ≥ 25 kg/m2 & < 30 kg/m2; Obese defined as BMI ≥ 30 kg/m2
Limitation in ADLs is present if participant has difficulty with one or more of the following activities of daily living: walking around the home, getting out of bed, eating, dressing, bathing, using the toilet
Limitation in iADLs is present if participant has difficulty with one or more of the following instrumental activities of daily living: heavy housework, light housework, shopping, preparing meals, paying bills, using the telephone
Frailty defined as frail if three or more of the following are present, pre-frail as 1–2 of the following are present, and not frail as none of the following are present: unintentional weight loss of ≥ 10 pounds in the past year, self-reported exhaustion, weak grip strength (lowest quintile) calculated as the mean of three serial measurements of the dominant hand adjusted for sex and BMI, low physical activity (lowest quintile) calculated as frequency and duration over the past 2 weeks from the Minnesota Leisure Time Activities Questionnaire (30), and low walking speed (lowest quintile) for timed walk over 4.57 meters or 15 feet at usual pace by height and sex (31)
Restricted to oral corticosteroid use
Defined as use of vitamin D, calcium, selective estrogen receptor modifiers (SERMs), estrogens, or bisphosphonates
Defined as use of loop diuretics, thiazide diuretics, selective serotonin reuptake inhibitors, anticonvulsants, benzodiazepines, sedatives/hypnotics, proton pump inhibitors, thiazolidinediones, or thyroid medications
Defined as servings per day of unprocessed red meat, processed meat, fish, chicken and eggs obtained from a food frequency questionnaire
Estimated glomerular filtration rate calculated from serum creatinine and Cystatin C using a combined equation (28)
3.2. TMAO and hip fracture
There were 666 incident hip fractures (515 in women, 151 in men) over a median 13.6 years of follow-up (range:0.01–26.0 years; 67,574 total person-years of follow-up). Per 100 person-years, hip fracture incidence was 0.99 (95% CI:0.82–1.18) overall, 1.17 (95% CI:1.02–1.33) in women, and 0.65 (95% CI:0.34–1.23) in men (Table 2). The hip fracture free survival curves did not significantly differ across TMAO quartiles in women or men (Figure 2). There were no significant associations between TMAO and hip fracture in either sex or overall in minimally or multivariable adjusted models (M0-M4) (Table 3).
Table 2.
Incidence of Hip Fracture by Baseline Trimethylamine N-oxide (TMAO) level in Quartiles
| Women | Men | Overall | ||||
|---|---|---|---|---|---|---|
| Hip fractures per Number at Risk, n | Incidence Rate (95% CI)a | Hip Fractures per Number at Risk, n | Incidence Rate (95% CI)a | Hip Fractures per Number at Risk, n | Incidence Rate (95% CI)a | |
| Quartile 1b | 134/775 | 1.17 (0.92, 1.49) | 39/480 | 0.61 (0.41, 0.89) | 173/1255 | 1.04 (0.57, 1.92) |
| Quartile 2c | 126/775 | 1.12 (0.88, 1.39) | 36/481 | 0.58 (0.37, 0.91) | 162/1256 | 0.93 (0.75, 1.34) |
| Quartile 3d | 126/774 | 1.14 (0.87, 1.49) | 40/480 | 0.70 (0.49, 1.01) | 162/1253 | 0.96 (0.77, 1.20) |
| Quartile 4e | 129/775 | 1.25 (0.91, 1.73) | 36/479 | 0.71 (0.06, 8.01) | 169/1255 | 1.02 (0.83, 1.26) |
| Total | 515/3099 | 1.17 (1.02, 1.33) | 151/1920 | 0.65 (0.34, 1.23) | 666/5019 | 0.99 (0.82, 1.18) |
Incidence rate is the number of hip fractures per 100 person-years. Incidence rates of hip fracture, total and across quartiles of TMAO, were calculated with quasi-Poisson model with offset to accommodate time at risk, unadjusted for covariates.
Quartile 1 of serum TMAO: 0.01 to 3.10 µmol/L in women; 0.45 to 3.56 µmol/L in men; 0.01 to 3.25 µmol/L overall
Quartile 2 of serum TMAO: 3.11 to 4.62 µmol/L in women; 3.57 to 5.24 µmol/L in men; 3.25 to 4.83 µmol/L overall
Quartile 3 of serum TMAO: 4.63 to 7.54 µmol/L in women; 5.25 to 8.43 µmol/L in men; 4.84 to 7.90 µmol/L overall
Quartile 4 of serum TMAO: 7.55 to 160.09 µmol/L in women; 8.44 to 254.99 µmol/L in men; 7.91 to 254.99 µmol/L overall
Figure 2.
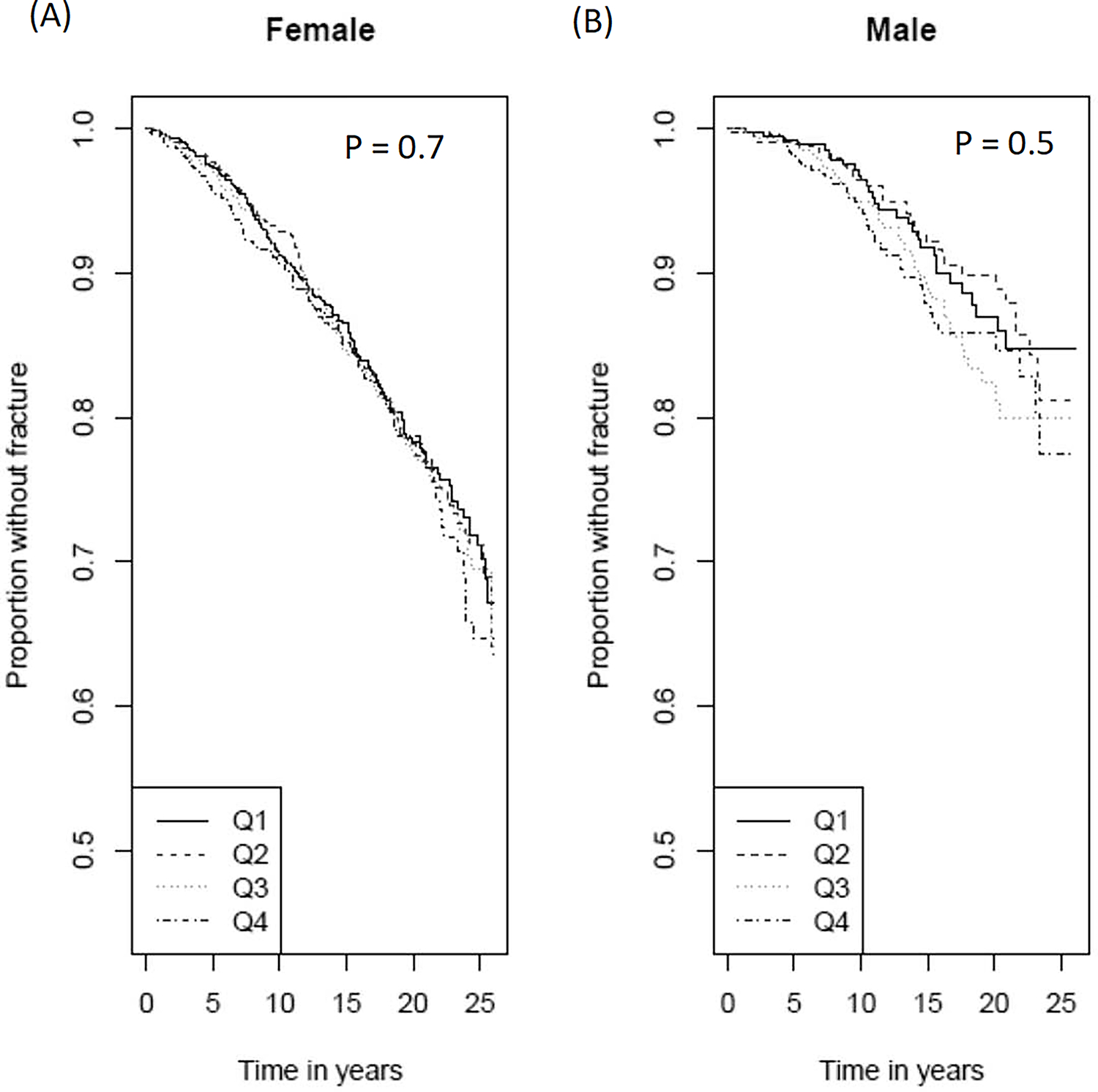
Sex-stratified Kaplan Meier Plots of Incident Hip Fracture in Participants by Trimethylamine N-oxide (TMAO) Quartile, in Women (A) and Men (B).
P-values displayed are from log-rank tests across quartiles of TMAO for each sex. Q1=first quartile; Q2=second quartile; Q3=third quartile; Q4=fourth quartile.
Table 3.
Associations between Trimethylamine N-oxide (TMAO) and Incident Hip Fracture, shown as Hazard Ratios of Incident Hip Fracture per Doubling of Baseline Log2-transformed TMAO level, Total and Stratified by Body Mass Index (BMI) Category
| Hazard Ratio and 95% Confidence Interval | |||||||
|---|---|---|---|---|---|---|---|
| Women (n=3099) | Men (n=1920) | Overall (5019) | |||||
| All Participants | Model 0a | 1.02 | (0.94, 1.10) | 1.10 | (0.95, 1.27) | 1.02 | (0.95, 1.10) |
| Model 1b | 1.00 | (0.92, 1.09) | 1.07 | (0.92, 1.25) | 1.01 | (0.94, 1.09) | |
| Model 2c | 1.00 | (0.91, 1.09) | 1.12 | (0.94, 1.32) | 1.02 | (0.94, 1.10) | |
| Model 3d | 1.00 | (0.92, 1.09) | 1.12 | (0.95, 1.33) | 1.02 | (0.94, 1.10) | |
| Model 4e | 1.00 | (0.91, 1.09) | 1.10 | (0.92, 1.31) | 1.02 | (0.94, 1.10) | |
| Normal weight or underweightf | Model 0a | 0.92 | (0.83, 1.03) | 1.04 | (0.84, 1.29) | 0.93 | (0.84, 1.03) |
| Model 1b | 0.91 | (0.82, 1.02) | 1.03 | (0.82, 1.30) | 0.93 | (0.84, 1.03) | |
| Model 2c | 0.88 | (0.78, 0.999) § | 0.98 | (0.74, 1.29) | 0.90 | (0.81, 1.01) | |
| Model 3d | 0.88 | (0.78, 1.00) | 0.99 | (0.75, 1.30) | 0.90 | (0.81, 1.01) | |
| Model 4e | 0.90 | (0.79, 1.02) | 1.00 | (0.75, 1.34) | 0.92 | (0.82, 1.03) | |
| Overweight or obeseg | Model 0a | 1.17 | (1.04, 1.32) § | 1.16 | (0.96, 1.40) | 1.16 | (1.05, 1.28) § |
| Model 1b | 1.14 | (1.01, 1.28) § | 1.11 | (0.91, 1.35) | 1.13 | (1.02, 1.25) § | |
| Model 2c | 1.15 | (1.02, 1.31) § | 1.23 | (0.98, 1.55) | 1.18 | (1.05, 1.31) § | |
| Model 3d | 1.15 | (1.01, 1.31) § | 1.23 | (0.98, 1.54) | 1.17 | (1.05, 1.31) § | |
| Model 4e | 1.13 | (0.99, 1.29) | 1.20 | (0.95, 1.52) | 1.15 | (1.03, 1.28) § | |
| Obeseh | Model 0a | 1.24 | (0.998, 1.55) | 0.77 | (0.44, 1.35) | 1.15 | (0.94, 1.42) |
| Model 1b | 1.22 | (0.98, 1.53) | 0.73 | (0.41, 1.29) | 1.13 | (0.92, 1.39) | |
| Model 2c | 1.37 | (1.08, 1.73) § | 2.56 | (0.84, 7.81) | 1.33 | (1.07, 1.65) § | |
| Model 3d | 1.37 | (1.08, 1.74) § | 3.10 | (1.08, 8.89) § | 1.33 | (1.07, 1.65) § | |
| Model 4e | 1.32 | (1.03, 1.70) § | 1.45 | (0.49, 4.29) | 1.25 | (0.99, 1.58) | |
Indicates statistical significance
Model 0: Unadjusted
Model 1: Adjusted for age, race, sex (overall only), study site, education level
Model 2: Adjusted for the covariates in Model 1 + smoking status, pack-year smoking history, alcohol use, prevalent diabetes, prevalent CVD, BMI, physical activity, presence of a limitation in ADLs, presence of a limitation in iADLs, frailty, osteoporosis medications (vitamin D, calcium, estrogens, selective estrogen receptor modifiers, bisphosphonates), oral corticosteroid use, and other medications affecting bone metabolism (loop diuretic, thiazide diuretic, SSRI, anticonvulsants, benzodiazepines, sedative/hypnotics, proton pump inhibitors, thiazolidinediones, and thyroid medications)
Model 3: Adjusted for the covariates in Model 2 + CRP and total animal source foods (unprocessed red meat, processed meat, fish, chicken and eggs)
Model 4: Adjusted for the covariates in Model 3 + eGFR
Normal weight or underweight defined as BMI < 25 kg/m2
Overweight or obese defined as BMI ≥ 25 kg/m2
Obese defined as BMI ≥ 30 kg/m2
Stratified by median total animal source food intake (1.6 servings/day), after multivariable adjustment, there was no significant association between TMAO and hip fracture in both women and men (Supplementary Table 4).
In exploratory analyses, a significant interaction was identified between BMI and the relationship of TMAO with hip fracture (P-interaction<0.01). Among participants with normal or underweight (BMI<25 kg/m2), there was no significant association between TMAO and hip fracture in either sex or overall in final multivariable adjusted models (M3) (Table 3). In participants with overweight or obesity (BMI≥25 kg/m2), in M3, higher TMAO was significantly associated with hip fracture hazard in women (HR per doubling of TMAO [95% CI]: 1.15 [1.01–1.31]) and overall (HR [95% CI]: 1.17 [1.05–1.31]), with a similar trend in men that did not reach statistical significance (HR [95% CI]: 1.23 [0.98–1.54]) (Table 3). In participants with obesity (BMI≥30 kg/m2), in M3, higher TMAO was also significantly associated with hip fracture hazard in women, in men, and overall (Table 3). The HR in participants with overweight or obesity was not appreciably changed in the exploratory model (M4) but may be attenuated in men with obesity.
3.3. TMAO and BMD
In the BMD cohort, there was no significant association between TMAO and total hip BMD in either sex or overall in final multivariable adjusted models (M3) or exploratory models (M4) (Table 4). In M3, there was no significant association between TMAO and BMD in any BMI category (Table 4).
Table 4.
Associations between Trimethylamine N-oxide (TMAO) and Total Hip Bone Mineral Density (BMD) (Grams per Square Centimeter × 100), shown as the β Estimate per Doubling of Baseline Log2-transformed TMAO level, Total and Stratified by Body Mass Index (BMI) Category
| Total hip BMD estimate (β) and 95% Confidence Interval | |||||||
|---|---|---|---|---|---|---|---|
| Women (n=873) | Men (n=527) | Overall (n=1400) | |||||
| All Participants | Model 0a | −0.25 | (−1.11, 0.60) | −1.31 | (−2.57,−0.05) § | 0.05 | (−0.78, 0.89) |
| Model 1b | 0.32 | (−0.45, 1.09) | −0.11 | (−1.30, 1.09) | 0.12 | (−0.54, 0.77) | |
| Model 2c | 0.41 | (−0.35, 1.17) | 0.20 | (−1.03, 1.42) | 0.28 | (−0.37, 0.94) | |
| Model 3d | 0.42 | (−0.34, 1.17) | 0.19 | (−1.04, 1.42) | 0.28 | (−0.37, 0.93) | |
| Model 4e | 0.48 | (−0.29, 1.26) | −0.19 | (−1.48, 1.09) | 0.22 | (−0.45, 0.90) | |
| Normal weight or underweightf | Model 0a | −0.14 | (−1.20, 0.92) | −0.14 | (−2.10, 1.83) | 0.38 | (−0.80,1.56) |
| Model 1b | 0.28 | (−0.74, 1.30) | 1.26 | (−0.68, 3.19) | 0.52 | (−0.42, 1.45) | |
| Model 2c | 0.26 | (−0.80, 1.32) | 1.09 | (−0.98, 3.16) | 0.54 | (−0.43, 1.51) | |
| Model 3d | 0.21 | (−0.84, 1.26) | 1.10 | (−0.98, 3.17) | 0.54 | (−0.43, 1.50) | |
| Model 4e | 0.29 | (−0.80, 1.38) | 0.84 | (−1.42, 3.10) | 0.55 | (−0.46, 1.56) | |
| Overweight or obeseg | Model 0a | −0.24 | (−1.37, 0.89) | −2.12 | (−3.65, −0.59) § | −0.17 | (−1.24, 0.90) |
| Model 1b | 0.21 | (−0.81, 1.23) | −1.21 | (−2.67, 0.25) | −0.33 | (−1.17, 0.51) | |
| Model 2c | 0.47 | (−0.61, 1.54) | −0.39 | (−1.98, 1.19) | 0.03 | (−0.85, 0.90) | |
| Model 3d | 0.50 | (−0.58, 1.57) | −0.35 | (−1.95, 1.24) | 0.02 | (−0.86, 0.90) | |
| Model 4e | 0.54 | (−0.57, 1.64) | −0.74 | (−2.39, 0.90) | −0.09 | (−0.998, 0.81) | |
| Obeseh | Model 0a | −0.37 | (−2.37, 1.63) | −4.75 | (−8.68, −0.83) § | −0.54 | (−2.56, 1.49) |
| Model 1b | 0.10 | (−1.78, 1.99) | −2.46 | (−6.16, 1.23) | −0.58 | (−2.24, 1.07) | |
| Model 2c | 0.21 | (−2.11, 2.52) | −2.55 | (−6.74, 1.65) | −0.61 | (−2.49, 1.27) | |
| Model 3d | 0.31 | (−1.96, 2.57) | −1.93 | (−6.27, 2.42) | −0.62 | (−2.49, 1.25) | |
| Model 4e | 0.79 | (−1.59, 3.16) | −3.31 | (−7.85, 1.22) | −0.59 | (−2.54, 1.36) | |
Indicates statistical significance
Model 0: Unadjusted
Model 1: Adjusted for age, race, sex (overall only), study site, education level
Model 2: Adjusted for the covariates in Model 1 + smoking status, pack-year smoking history, alcohol use, prevalent diabetes, prevalent CVD, BMI, physical activity, presence of a limitation in ADLs, presence of a limitation in iADLs, frailty, osteoporosis medications (vitamin D, calcium, estrogens, selective estrogen receptor modifiers, bisphosphonates), oral corticosteroid use, and other medications affecting bone metabolism and/or fracture risk (loop diuretic, thiazide diuretic, SSRI, anticonvulsants, benzodiazepines, sedative/hypnotics, proton pump inhibitors, thiazolidinediones, and thyroid medications)
Model 3: Adjusted for the covariates in Model 2 + CRP and total animal source foods (unprocessed red meat, processed meat, fish, chicken and eggs)
Model 4: Adjusted for the covariates in Model 3 + eGFR
Normal weight or underweight defined as BMI < 25 kg/m2
Overweight or obese defined as BMI ≥ 25 kg/m2
Obese defined as BMI ≥ 30 kg/m2
3.4. Change in TMAO and hip fracture
Assessing change in TMAO over time, the mean (SD) annualized change in TMAO was 0.04 (2.24) μmol/L (range:−21.77 to 40.99 μmol/L). There was a modest positive correlation between the baseline and follow-up log2TMAO (r=0.25; 95% CI:0.22–0.29) (Supplementary Figure 1), which did not significantly differ by sex (0.27 in women, 0.22 in men). There was no significant association between annualized change in log2TMAO and hip fracture in either sex or overall in minimally or multivariable adjusted models (M0-M4) (Supplementary Table 5). There was no significant interaction with BMI.
3.5. Sensitivity analyses
In sensitivity analysis using time-varying updating of TMAO measures and incorporating participants with TMAO measured only in 1996–1997, there was no significant association between TMAO and hip fracture overall or in either sex in minimally or multivariable adjusted models (M0-M4) (Supplementary Table 6). Our results were not appreciably changed by adjusting for grip strength (Supplemental Table 7), adjusting for history of falls (Supplemental Table 7) or considering time-varying weight as a covariate.
4. Discussion
In this large population of older, community-dwelling men and women, we did not find a significant association between plasma levels of the gut microbiota-derived metabolite TMAO and risk of hip fracture or total hip BMD. Dietary total animal source food intake was not identified as a mediator between TMAO and hip fracture. We found no significant association between annualized change in TMAO and risk of hip fracture. In exploratory analyses, higher TMAO was significantly associated with risk of hip fracture in participants with overweight and obesity.
Our results diverged from those of Liu et al (24) in their retrospective case-control study of 286 postmenopausal women showing higher TMAO was associated with increased odds of hip fracture after adjusting for covariates. Three important differences between Liu et al’s analysis and ours are (i) our cohort design, which eliminates the powerful selection bias inherent in retrospective control selection, especially use of hospital-based controls as in that study; (ii) our ability to control for additional important covariates including age (13–15), BMI (43), prevalent diabetes (44,45), physical activity (46), and frailty (47); and (iii) our use of a longitudinal design with TMAO measured before incident hip fracture. Racial or dietary population differences may account for varying results, but the TMAO interquartile ranges were similar, and our study did not find dietary total animal source food intake to mediate the association of TMAO and fracture. Our results were consistent with findings reported by Batteux et al, conducted in a smaller longitudinal study of patients on dialysis undergoing renal transplant (n=310), in which no association was found between TMAO at time of renal transplant and osteoporotic fracture risk (48). However, our study has far greater statistical power, greater generalizability to older adults, and longer follow-up.
Previous reports of the association of TMAO and BMD have yielded inconsistent results. In a very small, cross-sectional case-control study (n=10 cases), a significant inverse correlation was seen between TMAO and femoral neck BMD (22). Bias in control selection is a major limitation of such designs. Congruent with our findings, no significant association was identified between TMAO and total hip BMD in a post-hoc analysis of a randomized controlled trial of weight loss (n=264 participants with overweight or obesity) (25) or in Batteux et al’s study of renal transplant recipients (48). However, among the weight loss trial participants with overweight or obesity, Zhou et al reported decreased TMAO over time was associated with decreased spine and whole-body, but not total hip, BMD (25). Given these differential findings by Zhou et al in the association of change in TMAO and BMD based on site, and because our study was limited in scope to the hip, larger longitudinal studies evaluating the association of TMAO with clinical metrics of bone health at other osteoporotic fracture sites are warranted.
We found in exploratory analyses a 17% higher hazard of incident hip fracture per doubling of TMAO in participants with overweight or obesity (95% CI: 5% to 31%), with similar hazard estimates in both men and women. Generally similar findings were seen in participants with obesity in men, women and overall. Inflammation could play a role in this association. It has been hypothesized that TMAO may exert pro-osteoporotic effects by inducing oxidative stress which activates NF-κB signaling and the NLRP3 inflammasome (16–18,20–23). Obesity is clearly characterized by elevated oxidative stress and inflammation (36), which could make it fertile ground for additional influence of pro-inflammatory compounds like TMAO. In addition, while FMO3 converts trimethylamine to TMAO predominately in the liver, visceral adipose tissue expresses FMO3 (49). Inhibition of FMO3 stimulates white adipose tissue beiging and promotes resistance to obesity (49). Our findings suggest that the potential role of visceral fat in TMAO metabolism and TMAO-associated hip fracture risk, possibly through enhanced inflammation, merits further study.
This study is the largest study to evaluate the association of TMAO with incident hip fracture risk or BMD. Further, it is the only prospective study of community-dwelling older adults to examine the association of TMAO with incident hip fracture and the only prospective study to assess if change in TMAO is associated with incident hip fracture. The follow-up period was long, and we controlled for numerous potentially important covariates.
This study has several limitations. The primary analyses relied on a one-time assessment of TMAO. Diet and gut microbiome composition may have changed during the follow-up period, reflected in the only modest positive correlation between baseline and follow-up TMAO levels. However, results were not appreciably changed in sensitivity analyses with serial updating of TMAO. Our analyses of BMD were cross-sectional, and the time between TMAO and DXA measurements varied between participants and was long for all. Dietary data was imputed for the African American cohort. The number of hip fractures in men was low (n=151), and we may have lacked the power to examine the associations reported, particularly in BMI-stratified analyses. We lacked data on incident fractures at any other site beside the hip, and the association between TMAO and fracture may differ at other fracture sites. Data on factors associated with TMAO production (i.e., composition of the gut microbiome, FMO3 activity) were not collected.
In conclusion, our data suggested no significant association between TMAO at baseline or change in TMAO over time prospectively with hip fracture incidence in older, community-dwelling men and women. We likewise found no significant association cross-sectionally between TMAO and total hip BMD in older men or women. Our exploratory findings suggested there may be a weak association between TMAO and hip fracture in older adults with overweight or obesity.
Supplementary Material
Funding:
This work was supported by grant RO1HL135920 from the National Heart, Lung, and Blood Institute (NHLBI) and contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Abbreviations
- TMAO
trimethylamine N-oxide
- BMD
Bone mineral density
- CHS
Cardiovascular Health Study
- FMO3
Flavin-containing monooxygenase 3
- ROS
Reactive oxygen species
- NF-κB
nuclear factor κB
- NLRP3
nucleotide-binding oligomerization domain-like (NOD-like) receptor protein 3
- CVD
Cardiovascular disease
- ADL
activities of daily living
- iADL
instrumental activities of daily living
- CRP
C reactive protein
Footnotes
Disclosure Summary:
Drs Stanley Hazen and Zeneng Wang report being named as co-inventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Drs Hazen and Wang report having received royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab, a fully owned subsidiary of Quest Diagnostics, and Procter & Gamble. Dr. Hazen reports having been a paid consultant for Proctor & Gamble, and having received research funds from Proctor & Gamble, Pfizer Inc., and Roche Diagnostics.
The remaining authors have nothing to disclose.
References
- 1.Ding K, Hua F, Ding W. Gut Microbiome and Osteoporosis. Aging Dis. 2020;11(2):438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernandez CJ, Guss JD, Luna M, Goldring SR. Links Between the Microbiome and Bone. J Bone Miner Res. 2016;31(9):1638–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014;229(11):1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schepper JD, Collins F, Rios-Arce ND, Kang HJ, Schaefer L, Gardinier JD, Raghuvanshi R, Quinn RA, Britton R, Parameswaran N, McCabe LR. Involvement of the Gut Microbiota and Barrier Function in Glucocorticoid-Induced Osteoporosis. J Bone Miner Res. 2020;35(4):801–820. [DOI] [PubMed] [Google Scholar]
- 5.Lambert MNT, Thybo CB, Lykkeboe S, Rasmussen LM, Frette X, Christensen LP, Jeppesen PB. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial. Am J Clin Nutr. 2017;106(3):909–920. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson AG, Sundh D, Bäckhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. 2018;284(3):307–317. [DOI] [PubMed] [Google Scholar]
- 7.Jansson P-A, Curiac D, Lazou Ahrén I, Hansson F, Martinsson Niskanen T, Sjögren K, Ohlsson C. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet Rheumatology. 2019;1(3):e154–e162. [DOI] [PubMed] [Google Scholar]
- 8.Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17(1):49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Waiz M, Mitchell SC, Idle JR, Smith RL. The metabolism of 14C-labelled trimethylamine and its N-oxide in man. Xenobiotica. 1987;17(5):551–558. [DOI] [PubMed] [Google Scholar]
- 11.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J, Sutter JL, Caudill MA. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol Nutr Food Res. 2017;61(1). [DOI] [PubMed] [Google Scholar]
- 13.Brunt VE, Gioscia-Ryan RA, Casso AG, VanDongen NS, Ziemba BP, Sapinsley ZJ, Richey JJ, Zigler MC, Neilson AP, Davy KP, Seals DR. Trimethylamine-N-Oxide Promotes Age-Related Vascular Oxidative Stress and Endothelial Dysfunction in Mice and Healthy Humans. Hypertension. 2020;76(1):101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D, Ke Y, Zhan R, Liu C, Zhao M, Zeng A, Shi X, Ji L, Cheng S, Pan B, Zheng L, Hong H. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell. 2018;17(4):e12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, Senin U, Pacifici R, Cherubini A. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88(4):1523–1527. [DOI] [PubMed] [Google Scholar]
- 17.Basu S, Michaëlsson K, Olofsson H, Johansson S, Melhus H. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun. 2001;288(1):275–279. [DOI] [PubMed] [Google Scholar]
- 18.Callaway DA, Jiang JX. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab. 2015;33(4):359–370. [DOI] [PubMed] [Google Scholar]
- 19.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun X, Jiao X, Ma Y, Liu Y, Zhang L, He Y, Chen Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun. 2016;481(1–2):63–70. [DOI] [PubMed] [Google Scholar]
- 21.Ke Y, Li D, Zhao M, Liu C, Liu J, Zeng A, Shi X, Cheng S, Pan B, Zheng L, Hong H. Gut flora-dependent metabolite Trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic Biol Med. 2018;116:88–100. [DOI] [PubMed] [Google Scholar]
- 22.Lin H, Liu T, Li X, Gao X, Wu T, Li P. The role of gut microbiota metabolite trimethylamine N-oxide in functional impairment of bone marrow mesenchymal stem cells in osteoporosis disease. Ann Transl Med. 2020;8(16):1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Chen B, Zhu R, Li R, Tian Y, Liu C, Jia Q, Wang L, Tang J, Zhao D, Mo F, Liu Y, Li Y, Orekhov AN, Brömme D, Zhang D, Gao S. Fructus Ligustri Lucidi preserves bone quality through the regulation of gut microbiota diversity, oxidative stress, TMAO and Sirt6 levels in aging mice. Aging (Albany NY). 2019;11(21):9348–9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Guo YL, Meng S, Gao H, Sui LJ, Jin S, Li Y, Fan SG. Gut microbiota-dependent Trimethylamine N-Oxide are related with hip fracture in postmenopausal women: a matched case-control study. Aging (Albany NY). 2020;12(11):10633–10641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou T, Heianza Y, Chen Y, Li X, Sun D, DiDonato JA, Pei X, LeBoff MS, Bray GA, Sacks FM, Qi L. Circulating Gut Microbiota Metabolite Trimethylamine N-Oxide (TMAO) and Changes in Bone Density in Response to Weight Loss Diets: The POUNDS Lost Trial. Diabetes Care. 2019;42(8):1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. [DOI] [PubMed] [Google Scholar]
- 27.Psaty BM, Delaney JA, Arnold AM, Curtis LH, Fitzpatrick AL, Heckbert SR, McKnight B, Ives D, Gottdiener JS, Kuller LH, Longstreth WT Jr. Study of Cardiovascular Health Outcomes in the Era of Claims Data: The Cardiovascular Health Study. Circulation. 2016;133(2):156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins J, Hirsch C, Whitmer R, Cauley J, Harris T. The association of bone mineral density and depression in an older population. J Am Geriatr Soc. 2001;49(6):732–736. [DOI] [PubMed] [Google Scholar]
- 29.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):270–277. [DOI] [PubMed] [Google Scholar]
- 30.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43(1):52–58. [PubMed] [Google Scholar]
- 32.Taylor HL, Jacobs DR Jr., Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–755. [DOI] [PubMed] [Google Scholar]
- 33.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 34.Cawthon PM. Gender differences in osteoporosis and fractures. Clin Orthop Relat Res. 2011;469(7):1900–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porcelli T, Maffezzoni F, Pezzaioli LC, Delbarba A, Cappelli C, Ferlin A. MANAGEMENT OF ENDOCRINE DISEASE: Male osteoporosis: diagnosis and management - should the treatment and the target be the same as for female osteoporosis? Eur J Endocrinol. 2020;183(3):R75–r93. [DOI] [PubMed] [Google Scholar]
- 36.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–590. [DOI] [PubMed] [Google Scholar]
- 37.Albrand G, Munoz F, Sornay-Rendu E, DuBoeuf F, Delmas PD. Independent predictors of all osteoporosis-related fractures in healthy postmenopausal women: the OFELY study. Bone. 2003;32(1):78–85. [DOI] [PubMed] [Google Scholar]
- 38.Huang P, Luo K, Xu J, Huang W, Yin W, Xiao M, Wang Y, Ding M, Huang X. Sarcopenia as a Risk Factor for Future Hip Fracture: A Meta-Analysis of Prospective Cohort Studies. J Nutr Health Aging. 2021;25(2):183–188. [DOI] [PubMed] [Google Scholar]
- 39.Gadelha AB, Neri SGR, Oliveira RJ, Bottaro M, David AC, Vainshelboim B, Lima RM. Severity of sarcopenia is associated with postural balance and risk of falls in community-dwelling older women. Exp Aging Res. 2018;44(3):258–269. [DOI] [PubMed] [Google Scholar]
- 40.Crandall CJ, Yildiz VO, Wactawski-Wende J, Johnson KC, Chen Z, Going SB, Wright NC, Cauley JA. Postmenopausal weight change and incidence of fracture: post hoc findings from Women’s Health Initiative Observational Study and Clinical Trials. Bmj. 2015;350:h25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen Z, Yu C, Guo Y, Bian Z, Wei Y, Du H, Yang L, Chen Y, Gao Y, Zhang X, Chen J, Chen Z, Lv J, Li L. Weight loss since early adulthood, later life risk of fracture hospitalizations, and bone mineral density: a prospective cohort study of 0.5 million Chinese adults. Arch Osteoporos. 2020;15(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.R Development Core Team. R: The R Project for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 43.Dehghan P, Farhangi MA, Nikniaz L, Nikniaz Z, Asghari-Jafarabadi M. Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) potentially increases the risk of obesity in adults: An exploratory systematic review and dose-response meta-analysis. Obes Rev. 2020;21(5):e12993. [DOI] [PubMed] [Google Scholar]
- 44.Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118(4):476–481. [DOI] [PubMed] [Google Scholar]
- 45.Zhou L, Li X, Li S, Wen X, Peng Y, Zhao L. Relationship between dietary choline intake and diabetes mellitus in the National Health and Nutrition Examination Survey 2007–2010. J Diabetes. 2020. [DOI] [PubMed] [Google Scholar]
- 46.Argyridou S, Bernieh D, Henson J, Edwardson CL, Davies MJ, Khunti K, Suzuki T, Yates T. Associations between physical activity and trimethylamine N-oxide in those at risk of type 2 diabetes. BMJ Open Diabetes Res Care. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He W, Luo Y, Liu JP, Sun N, Guo D, Cui LL, Zheng PP, Yao SM, Yang JF, Wang H. Trimethylamine N-Oxide, a Gut Microbiota-Dependent Metabolite, is Associated with Frailty in Older Adults with Cardiovascular Disease. Clin Interv Aging. 2020;15:1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batteux B, Bodeau S, André C, Hurtel-Lemaire AS, Gras-Champel V, Desailly-Henry I, Masmoudi K, Bennis Y, Massy ZA, Kamel S, Choukroun G, Liabeuf S. Association between Uremic Toxin Concentrations and Bone Mineral Density after Kidney Transplantation. Toxins (Basel). 2020;12(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schugar RC, Shih DM, Warrier M, Helsley RN, Burrows A, Ferguson D, Brown AL, Gromovsky AD, Heine M, Chatterjee A, Li L, Li XS, Wang Z, Willard B, Meng Y, Kim H, Che N, Pan C, Lee RG, Crooke RM, Graham MJ, Morton RE, Langefeld CD, Das SK, Rudel LL, Zein N, McCullough AJ, Dasarathy S, Tang WHW, Erokwu BO, Flask CA, Laakso M, Civelek M, Naga Prasad SV, Heeren J, Lusis AJ, Hazen SL, Brown JM. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017;19(12):2451–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


