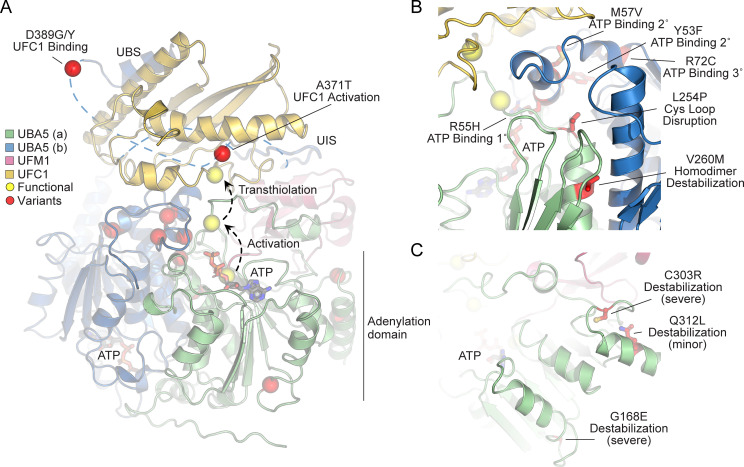
Figure 5. Structural analysis of UBA5 variants.
(A) Composite model of a UBA5 homodimer (green and blue) bound to ATP (gray sticks), ubiquitin fold modifier 1 (UFM1; magenta), and UFC1 (gold). The model was built using a series of UBA5 complex structures with UFM1 and UFC1 (PDB 6H77, 7NW1, and a modeled UBA5:UFC1 complex; Kumar et al., 2021; Soudah et al., 2019). Functional residues comprising the active site cysteines of UBA5 and UFC1, as well as the C-terminus of UFM1 are shown in yellow spheres. UBA5 variants are shown in red spheres and are labeled with their predicted structural effects. (B) Close-up view of variants (red sticks) within the UBA5 active site (yellow sphere), ATP-binding pocket, and homodimerization interface. (C) Close-up view of variants (red sticks) expected to impact UBA5 protein stability (results shown in the following figures).