Figure 6. Preparation and stability of UBA5 variant proteins.
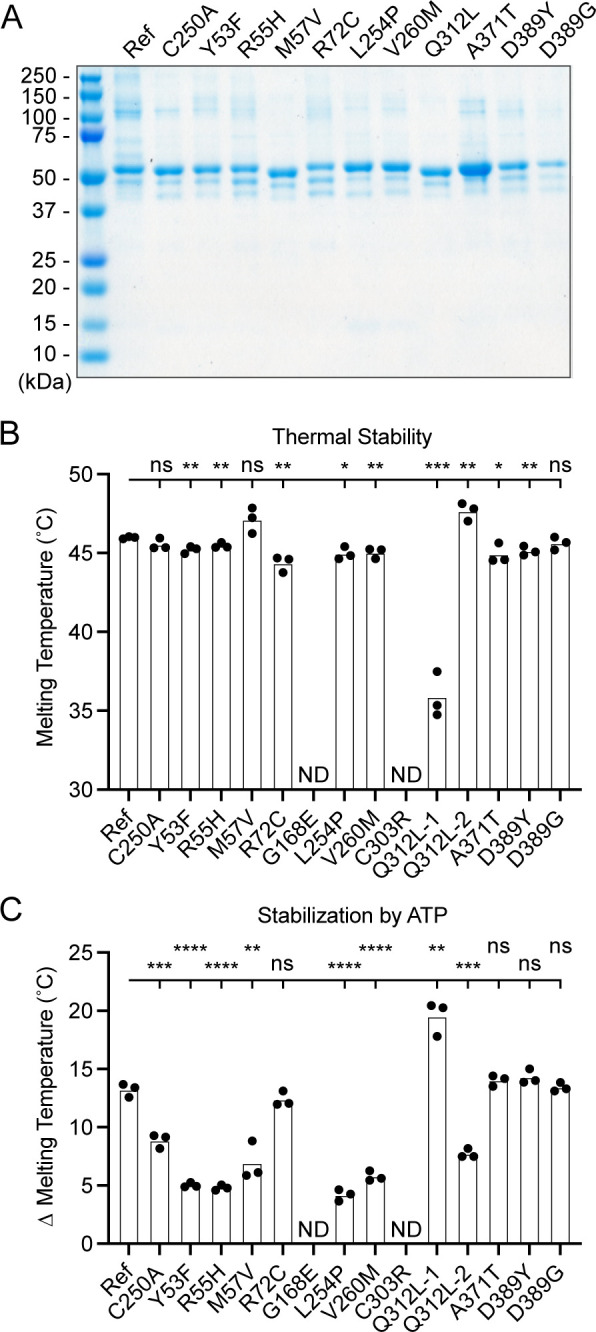
(A) Coomassie-stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) analysis of all purified UBA5 variant proteins. (B) Thermal shift assay measuring the melting temperature (Tm) of all UBA5 variant proteins, with the exception of p.Gly168Glu and p.Cys303Arg which could not be produced. The p.Gln312Leu variant displayed two melting curves. Experiments were performed in triplicate over three biological replicates. (C) Change in melting temperature for all UBA5 variants in the presence of 5 mM ATP. Upon ATP addition, the p.Gln312Leu variant transitioned to a single melting curve. Experiments were performed in triplicate over three biological replicates. (B, C) Statistical analyses were performed via unpaired Student’s t-test. n = 3 biological replicates. ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
