Abstract
The growth rates of Pseudomonas putida KT2442 and mt-2 on benzoate, 4-hydroxybenzoate, or 4-methylbenzoate showed an exponential decrease with decreasing oxygen tensions (partial O2 tension [pO2] values). The oxygen tensions resulting in half-maximal growth rates were in the range of 7 to 8 mbar of O2 (corresponding to 7 to 8 μM O2) (1 bar = 105 Pa) for aromatic compounds, compared to 1 to 2 mbar for nonaromatic compounds like glucose or succinate. The decrease in the growth rates coincided with excretion of catechol or protocatechuate, suggesting that the activity of the corresponding oxygenases became limiting. The experiments directly establish that under aerobic and microaerobic conditions (about 10 mbar of O2), the diffusion of O2 into the cytoplasm occurs at high rates sufficient for catabolic processes. This is in agreement with calculated O2 diffusion rates. Below 10 mbar of O2, oxygen became limiting for the oxygenases, probably due to their high Km values, but the diffusion of O2 into the cytoplasm presumably should be sufficiently rapid to maintain ambient oxygen concentrations at oxygen tensions as low as 1 mbar of O2. The consequences of this finding for the availability of O2 as a substrate or as a regulatory signal in the cytoplasm of bacterial cells are discussed.
During aerobic growth, bacteria consume O2 at high rates. The consumption of O2 by oxidases takes place on the cytoplasmic side of the membrane. Since the diffusion of O2 across the membrane is rapid, the supply of the oxidases with O2 is guaranteed even at the very low O2 tensions which are sufficient for aerobic growth (<1 mbar of O2) (2, 4, 15, 16). Previously, the rate of O2 diffusion into the cytoplasm of Escherichia coli was calculated from the cell dimensions and the diffusion coefficients and compared to the rates of O2 consumption (2, 21, 22). It was estimated that at O2 tensions as low as 0.2 mbar of O2 (corresponding to 0.2 μM O2), the supply of O2 by diffusion exceeds the consumption by respiration. In agreement with this calculation, in E. coli the fermentation pathways were synthesized and used only at partial O2 tension (pO2) values well below 1 mbar of O2 (3). Thus, O2 is able to reach the active sites of the oxidases at rates sufficient to support aerobic respiration even at very low O2 tensions.
The O2 supply of the cytoplasmic space is not known and might be different from that of the membrane where the oxidases are located. From the diffusional parameters and the cell dimensions, it was calculated that the concentrations of O2 should be the same within and outside the bacteria at O2 tensions as low as 1 mbar of O2 (21, 22). Therefore, we aimed for an experimental proof of the availability of O2 in the bacterial cytoplasm under aerobic and microaerobic conditions.
For the degradation of aromatic compounds like benzoate, oxygenases are required for oxidative cleavage of the aromatic ring (7, 10). Due to the cytoplasmic location of the oxygenases and the need for molecular oxygen as a cosubstrate, the turnover of aromatic compounds depends on the availability of O2 in the cytoplasm. The rate of metabolism of aromatic compounds therefore provides information on the minimal rate of O2 diffusion into the cytoplasm. To this end, the relation of metabolism of various aromatic compounds to the pO2 of the medium was studied. Pseudomonas putida KT2442 degrades benzoate by benzoate-1,2-dioxygenase and catechol-1,2-dioxygenase (ortho pathway), whereas 4-hydroxybenzoate is degraded via 4-hydroxybenzoate monooxygenase and protocatechuate-3,4-dioxygenase (ortho cleavage). 4-Methylbenzoate is metabolized by P. putida mt-2 by toluate-1,2-dioxygenase and catechol-2,3-dioxygenase (meta cleavage) (5, 8). The Km values for O2 of the oxygenases (≥7 μM) (1, 6, 12, 13) are much higher than those of the oxidases (<0.1 μM) (4, 15, 16). Therefore, limitation of growth or catabolism by O2 must be due to the oxygenases, and information on O2 diffusion into the cytoplasm and the O2 concentration in the cytoplasm can be drawn from the growth-limiting pO2 values. Here we report on experimental proof of the availability of O2 in the cytoplasm. This finding also provides a basis for our understanding of the O2 sensing by cytoplasmic O2 sensor proteins like FNR (fumarate nitrate reductase regulator) from E. coli (9, 19, 22, 23) and the homologous proteins from Pseudomonas (17, 25) which are supposed to react directly with O2 in the cytoplasm (2, 22, 23).
MATERIALS AND METHODS
Bacteria and media.
P. putida KT2442 and P. putida mt-2(pWWO) were provided by I. Wagner-Döbler (Braunschweig, Germany) and M. Schlömann (Stuttgart-Hohenheim, Germany) (5, 24). P. putida KT2442 was grown in a modified M9 mineral medium (pH adjusted to 7.1) supplemented with a mineral salts solution and with glucose, succinate, benzoate, or 4-hydroxybenzoate (10 mM each) as sources of carbon and energy. The mineral salts solution was a combination of the following: solution 1, containing 25.39 g of MgCl2, 2.0 g of CaCO3, 4.5 g of FeSO4 · 7H2O, 0.85 g of MnSO4 · H2O, 1.44 g of ZnSO4 · 7H2O, 0.25 g of CuSO4 · 5H2O, 0.16 g of CaSO4 · 0.5H2O, and 0.02 g of H3BO3 dissolved in 51.3 ml of concentrated HCl and with water added to 100 ml; solution 2, containing 360 mM FeSO4 · 7H2O; and solution 3, containing 1 M MgSO4. Solutions 1 and 2 were filter sterilized, and solution 3 was autoclaved. Then 50 ml of solution 1, 2.5 ml of solution 2, 25 ml of solution 3, and 22.5 ml of autoclaved H2O were combined. The medium was supplemented with 0.25 ml of the resulting mineral salts solution per 100 ml. P. putida mt-2(pWWO) was grown in a phosphate-buffered medium (14.0 g of Na2HPO4 · 12H2O, 2.0 g of KH2PO4 per liter) supplemented with a salts solution (20 ml/liter of medium) containing 2.5 g of Ca(NO3) · 4H2O (autoclaved separately) per liter, 0.5 g of Fe(III)NH4-citrate per liter, 10 g of MgSO4 · 7H2O per liter, 50 g of (NH4)2SO4 per liter, and 50 ml of the Pfennig SL6 metal salts solution (14) per liter. The C source for P. putida mt-2 was 4-methylbenzoate (10 mM). E. coli MC4100 (18) was grown in M9 medium (11) supplemented with an amino acid mixture (20) and glucose (10 mM) or succinate (10 mM).
Growth.
P. putida was grown at 30°C. Growth under anaerobic conditions was performed in sealed bottles under an atmosphere of nitrogen (2, 3). For aerobic conditions, the bacteria were grown in Erlenmeyer flasks filled to within 10% of the maximal volume under vigorous shaking (3). The medium was inoculated from cultures grown overnight under aerobic conditions in the mineral medium (same C source as that in the main culture) to an A578 not higher than 0.06.
Growth in an oxystat.
Growth at defined O2 tensions (pO2) was performed in an oxystat (chemostat with constant pO2) (Biostat MD; Braun, Melsungen, Germany) in batch culture (1.5 liters) with constant stirring (400 rpm) (2, 3). The pO2 value of the medium was measured continuously with an O2 electrode. The pO2 was maintained at a constant level by supplying air (valve I) and N2 (valve II) to the vessel. When the pO2 fell below 98% of the set value, valve I opened and sterile air was supplied till the set value was reached. The flow of air was increased manually from about 0.16 to 1.6 liters min−1 during growth to compensate for the increasing O2 consumption. The flow of N2 (0.1 liters min−1) was decreased or switched off as required. E. coli was grown in the oxystat in the supplemented M9 medium as described previously (2, 3). Growth rates were calculated from μ = ln (A578,t2/A578,t1) · (t2 − t1)−1, where t2 and t1 are the times of measurement and A578,t1 and A578,t2 are the absorbance values at 578 nm measured at t1 and t2, respectively.
Analytical procedures.
Substrates (glucose, succinate, benzoate, 4-hydroxybenzoate, and 4-methylbenzoate) and products (catechol, protocatechuate) were determined from the supernatants of the cultures after removal of the bacteria by centrifugation. The substances were analyzed by high-performance liquid chromatography (HPLC) on an Aminex HPX87H column (300 by 7.8 mm; Bio-Rad) with 6.5 mM H2SO4 as the eluent (flow rate, 0.55 ml min−1) as described previously (20). The following substrates and products were determined and quantified with standard solutions by a refractive index and by a UV light detector (215 nm): glucose, glycerol, acetate, ethanol, formate, pyruvate, fumarate, succinate, and lactate. Benzoate (retention time [Rt] = 68 min), 4-hydroxybenzoate (Rt = 51 min), catechol (Rt = 32.0 min), and protocatechuate (Rt = 33.3 min) were identified by the Rt values of authentic substances, and the ratio of the refractive index/UV absorption at 215 nm was used to confirm the identities.
RESULTS AND DISCUSSION
Growth of P. putida on aromatic compounds at limiting pO2.
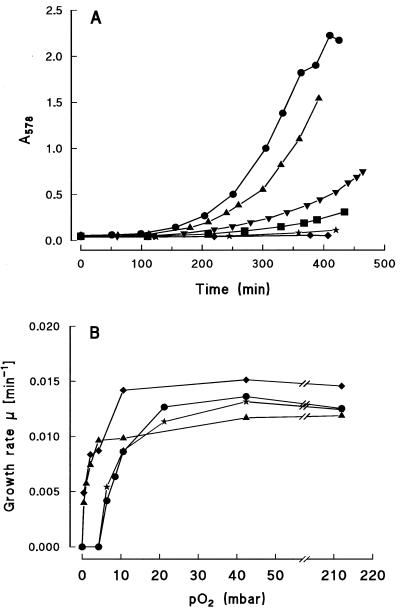
P. putida was grown on nonaromatic and aromatic substrates like glucose, succinate, and benzoate in an oxystat at defined pO2 values. In the oxystat, the set pO2 values could be maintained constant for the duration of the growth experiment. With glucose or succinate as the substrate, the growth behavior changed only when the pO2 fell below 10 mbar of O2 (corresponding to about 10 μM O2). At lower pO2 values, the growth rate and final cell density decreased, and under anaerobic conditions, no growth was observed. With benzoate or 4-hydroxybenzoate as the substrate, under aerobic conditions (212 mbar of O2; air saturation), growth of P. putida (Fig. 1A) was similar to that on glucose or succinate. However, with decreasing pO2 values, growth rate and yield decreased significantly.
FIG. 1.
Growth (A) and growth rates (B) of P. putida KT2442 grown in an oxystat on aromatic and nonaromatic substrates as a function of pO2. (A) Growth with benzoate in the oxystat at different pO2 values. Growth was performed in the mineral medium with 10 mM benzoate at 212 (•), 21 (▴), 8 (▾), 6 (▪), 4 (★), and 0 (⧫) mbar of O2. (B) The rate constants for growth (μ) were determined from the growth curves shown in panel A. Substrates (10 mM each) for growth: benzoate (•), 4-hydroxybenzoate (★), glucose (⧫), and succinate (▴).
In Fig. 1B, the rate constants for growth on aromatic and nonaromatic substrates are plotted versus the pO2 values. With glucose and succinate, growth of P. putida commenced at very low pO2 values and showed a saturation curve with increasing pO2. With the aromatic substrates benzoate and 4-hydroxybenzoate, growth started only at pO2 values above 4.2 mbar. With 4-methylbenzoate, the O2 requirement was even higher (data not shown). The maximal growth rates for succinate and benzoate corresponded to doubling times of 46 and 51 min, respectively. When E. coli was grown on succinate or glucose, the growth rates increased immediately from 0 mbar, similar to the growth rates of P. putida on the same substrates (data not shown). For growth on glucose, however, the growth rates did not drop to zero at 0 mbar of O2 due to the presence of fermentative growth. Thus, the growth rate at 0 mbar of O2 (μ = 0.011 min−1) was about half that of E. coli grown under aerobic conditions on glucose (μ = 0.020 min−1).
pO0.5 values for growth on aromatic substrates are higher than those for growth on nonaromatic substrates.
For P. putida, from the relation of the growth rates to the pO2 values, the pO0.5 values for the substrates can be determined. The pO0.5 value corresponds to the pO2 value yielding half-maximal growth rates (2, 3). The measured pO0.5 values can be classified into two groups. For growth of P. putida and E. coli on glucose and succinate, low values (pO0.5 ≤ 2 mbar of O2) were found. For growth on aromatic compounds, the pO0.5 values were distinctly higher and corresponded to about 8 mbar for growth on benzoate and 4-hydroxybenzoate and to 19 mbar for growth on 4-methylbenzoate.
Excretion of intermediates under O2 limitation.
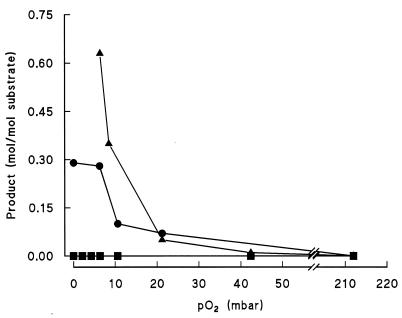
The growth medium was analyzed for products or intermediates excreted by the bacteria during growth in the oxystat at different pO2 values (Fig. 2). The medium was analyzed by HPLC for the presence of organic acids, alcohols, sugars, and aromatic compounds, in particular for intermediates of the respective metabolic routes. During growth at high oxygen tensions, all types of substrates were completely oxidized by P. putida and no organic end products were detected in significant amounts (>0.05 mol/mol of substrate). From glucose and succinate, no end products were excreted even at decreased oxygen tensions, indicating complete oxidation. When P. putida KT2442, however, was grown on benzoate, a product was found in the medium at oxygen tensions below 20 mbar which was identified as catechol. Catechol is an intermediate of the ortho cleavage pathway of benzoate. Up to 0.65 mol of catechol per mol of benzoate was measured, indicating a severe limitation in the ortho cleavage pathway resulting in the excretion of the intermediate without complete oxidation. During growth on 4-hydroxybenzoate, protocatechuate was excreted in large amounts (0.28 mol/mol of 4-hydroxybenzoate) at oxygen tensions below 20 mbar. Obviously, limitation of protocatechuate-3,4-dioxygenase activity (6) by low O2 tensions causes accumulation and excretion of the intermediate protocatechuate. Therefore, in both pathways, central steps, i.e., the dioxygenases reacting on catechol and protocatechuate, are limiting under microaerobic conditions.
FIG. 2.
Products in the culture medium of P. putida KT2442 excreted after growth at various pO2 values in the oxystat: catechol (▴) excreted during growth on benzoate (10 mM), protocatechuate (•) excreted during growth on 4-hydroxybenzoate, and end products (▪) from succinate and glucose. For end products tested, see Materials and Methods.
Availability of O2 as an intracellular substrate for aromatic substrate degradation.
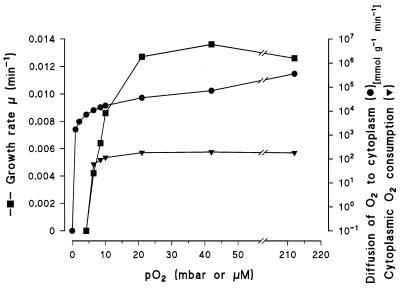
The data can be used to roughly estimate the rate of O2 diffusion into the cells required for this process. The rate of O2 consumption by the oxygenases in the cell interior (νO2in) is twice the rate of benzoate metabolism (νbenzoate) (Table 1) corresponding to 0.22 mmol of benzoate · min−1 · g (dry weight)−1 and 0.44 mmol of O2 · min−1 · g (dry weight)−1. The calculated rate of O2 diffusion into the cells under aerobic conditions, 360 mmol of O2 · min−1 · g (dry weight)−1 (Table 1), exceeds the rate of intracellular O2 consumption by the oxygenases by 3 orders of magnitude.
TABLE 1.
Metabolic and energetic parameters for growth of P. putida on benzoate
| Parametera or data | Value | Reference or source |
|---|---|---|
| Experimental parameters | ||
| Maximal growth rate on benzoate (μmax) (min−1) | 0.0136 | Fig. 1B |
| Molar growth yield on benzoate (Ybenzoate) [g (dw) · mol−1] | 62 | Fig. 1A |
| pO0.5 for half-maximal growth rate (μM) | 8.2 | This work |
| Km (catechol-1,2-dioxygenase) (μM O2) | 20 | 1, 12 |
| Calculated data [mmol · g (dw)−1] | ||
| Benzoate consumption at μmax (νbenzoate) | 0.22 | νbenzoate = μ/Ybenzoate |
| Intracellular O2 consumption (oxygenases, νO2in) | 0.44 | νO2in = 2 · νbenzoate |
| Intracellular O2 consumption (oxygenases plus oxidases, νO2in, t) | 1.65 | νO2in, t = 7.5 · νbenzoateb |
| Maximal rate of O2 diffusion into P. putida cells | 360 | 2, 21 |
g (dw), grams (dry weight).
Based on the growth reaction (1 benzoate + 7.5O2→7CO2 + 3H2O).
Plotting the rates of growth on benzoate as a function of the pO2 shows that diffusion of O2 is not limiting under aerobic or microaerobic conditions (Fig. 3): growth is limited apparently only at pO2 values below 10 mbar of O2. The growth limitation coincides with the excretion of the oxygenase substrates catechol and protocatechuate, demonstrating that oxygenation is the growth-limiting step. At 10 mbar of O2, the calculated diffusion is still higher by 2 orders of magnitude than the O2 consumption by the oxygenases (Fig. 3). Therefore, the decrease in the growth rate is presumably not caused by limiting O2 diffusion but by the high Km value (20 μM) of the oxygenase (Table 1). Thus, at pO2 values as low as 10 mbar, there is substantial O2 present in the cytoplasm. The high Km values of the oxygenases prevented an analysis of the situation at lower pO2 values. The calculation of the diffusion rates for O2, however, also suggests that at distinctly lower oxygen tensions, down to 1 mbar of O2, the intracellular pO2 equals the extracellular pO2 (2, 21, 22). The O2 present under aerobic and microaerobic conditions most likely is also used as the signal for O2 sensor-regulator proteins like FNR from E. coli (9, 22) and homologous proteins from Pseudomonas strains (17, 25) which are thought to react directly with O2 in the cytoplasm (2, 23). The regulatory pO0.5 which causes a switch from active (anaerobic) to inactive (aerobic) FNR is in the range of 1 to 5 mbar of O2 in the external medium for many target genes, which is in good agreement with the results found in the present work.
FIG. 3.
Growth rate, O2 consumption by dioxygenases, and maximal rate of O2 diffusion into the bacteria (P. putida KT2442) as a function of the pO2 in the medium during growth on benzoate. The growth rate was determined as described in the legend to Fig. 1. The O2 consumption by the dioxygenases was calculated from μ and from the molar growth yield on benzoate (Ybenzoate) under the respective conditions as shown in Table 1. The rate of diffusion into the bacteria was calculated as a function of external oxygen concentration (with the internal concentration set at zero). For the calculation, the diffusion coefficients of O2 in water, phospholipid, and cytoplasm and the cell dimensions were used (2, 22).
ACKNOWLEDGMENTS
We are grateful to Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie for financial support, to D. Vlad for HPLC analysis, and to I. Wagner-Döbler (Braunschweig, Germany) and M. Schlömann (Stuttgart-Hohenheim, Germany) for supplying strains.
REFERENCES
- 1.Barman T E. Enzyme handbook. Vol. 1. Berlin, Germany: Springer-Verlag KG; 1969. pp. 237–238. [Google Scholar]
- 2.Becker S, Holighaus G, Gabrielczyk T, Unden G. O2 as the regulatory signal for FNR-dependent gene regulation in Escherichia coli. J Bacteriol. 1996;178:4515–4521. doi: 10.1128/jb.178.15.4515-4521.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker S, Vlad D, Schuster S, Pfeiffer P, Unden G. Regulatory O2 tensions for the synthesis of fermentation products in Escherichia coli and relation to aerobic respiration. Arch Microbiol. 1997;168:290–296. doi: 10.1007/s002030050501. [DOI] [PubMed] [Google Scholar]
- 4.D’mello R, Hill S, Poole R K. The cytpochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two oxygen-binding haems: implications for regulation of activity in vivo by oxygen inhibition. Microbiology. 1996;142:755–763. doi: 10.1099/00221287-142-4-755. [DOI] [PubMed] [Google Scholar]
- 5.Franklin F C H, Bagdasarin M, Bagdasarin M M, Timmis K N. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc Natl Acad Sci USA. 1981;78:7458–7462. doi: 10.1073/pnas.78.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujisawa H. Protocatechuate 3,4-dioxygenase (Pseudomonas) Methods Enzymol. 1970;17A:526–529. [Google Scholar]
- 7.Harayama S, Kok M. Functional and evolutionary relationships among diverse oxygenases. Annu Rev Microbiol. 1992;46:565–601. doi: 10.1146/annurev.mi.46.100192.003025. [DOI] [PubMed] [Google Scholar]
- 8.Heuer H, Dwyer D F, Timmis K N, Wagner-Döbler I. Efficacy in aquatic microcosms of a genetically engineered pseudomonad applicable for bioremediation. Microb Ecol. 1995;29:203–220. doi: 10.1007/BF00167165. [DOI] [PubMed] [Google Scholar]
- 9.Lazazzera B A, Beinert H, Khoroshilova N, Kennedy M C, Kiley P J. DNA-binding and dimerization of the Fe-S-containing FNR protein from Escherichia coli are regulated by oxygen. J Biol Chem. 1996;271:2762–2768. doi: 10.1074/jbc.271.5.2762. [DOI] [PubMed] [Google Scholar]
- 10.Mason J R, Cammack R. The electron transport proteins of hydroxylating bacterial dioxygenases. Annu Rev Microbiol. 1992;46:277–305. doi: 10.1146/annurev.mi.46.100192.001425. [DOI] [PubMed] [Google Scholar]
- 11.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1992. [Google Scholar]
- 12.Nakazawa T, Nakazawa A. Pyrocatechase (Pseudomonas) Methods Enzymol. 1970;17A:518–522. [Google Scholar]
- 13.Nozaki M. Metapyrocatechase (Pseudomonas) Methods Enzymol Vol. 1970;17A:522–525. [Google Scholar]
- 14.Pfennig N, Lippert K D. Über das Vitamin B12-Bedürfnis phototropher Schwefelbakterien. Arch Microbiol. 1966;55:245–256. [Google Scholar]
- 15.Preisig O, Zuffrey R, Thöny-Meyer L, Appleby C A, Hennecke H. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J Bacteriol. 1996;178:1532–1538. doi: 10.1128/jb.178.6.1532-1538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice C W, Hempfling W P. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol. 1978;134:115–124. doi: 10.1128/jb.134.1.115-124.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawers R G. Identification and molecular characterization of a transcriptional regulator from Pseudomonas aeruginosa PAO1 exhibiting structural and functional similarity to the FNR protein of Escherichia coli. Mol Microbiol. 1991;5:1469–1481. doi: 10.1111/j.1365-2958.1991.tb00793.x. [DOI] [PubMed] [Google Scholar]
- 18.Silhavy T J, Berman M, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 19.Spiro S, Guest J R. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol Rev. 1990;75:399–428. doi: 10.1111/j.1574-6968.1990.tb04109.x. [DOI] [PubMed] [Google Scholar]
- 20.Tran Q H, Bongaerts J, Vlad D, Unden G. Requirement for the proton-pumping NADH dehydrogenase I of Escherichia coli in NADH→fumarate respiration and bioenergetic implications. Eur J Biochem. 1997;244:155–160. doi: 10.1111/j.1432-1033.1997.00155.x. [DOI] [PubMed] [Google Scholar]
- 21.Unden G, Becker S, Bongaerts J, Schirawski J, Six S. Oxygen regulated gene expression in facultatively anaerobic bacteria. Antonie Leeuwenhoek. 1994;66:3–23. doi: 10.1007/BF00871629. [DOI] [PubMed] [Google Scholar]
- 22.Unden G, Becker S, Bongaerts J, Holighaus G, Schirawski J, Six S. O2-sensing and O2-dependent gene regulation in facultatively anaerobic bacteria. Arch Microbiol. 1995;164:81–90. [PubMed] [Google Scholar]
- 23.Unden G, Schirawski J. The oxygen-responsive transcriptional regulator FNR of Escherichia coli: the search for signals and reactions. Mol Microbiol. 1997;25:205–210. doi: 10.1046/j.1365-2958.1997.4731841.x. [DOI] [PubMed] [Google Scholar]
- 24.Wagner-Döbler I, Pipke R, Timmis K N, Dwyer D F. Evaluation of aquatic sediment microcosms and their use in assessing effects on introduced microorganisms on ecosystem parameters. Appl Environ Microbiol. 1992;58:1249–1258. doi: 10.1128/aem.58.4.1249-1258.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmermann A, Reimmann C, Galimand M, Haas D. Anaerobic growth and cyanide synthesis of Pseudomonas aeruginosa depend on anr, a regulatory gene homologous with fnr of Escherichia coli. Mol Microbiol. 1991;5:1483–1490. doi: 10.1111/j.1365-2958.1991.tb00794.x. [DOI] [PubMed] [Google Scholar]