Figure 5.
Functional Role of N-Myristoylation of MARCKS in Cardiac Myocytes
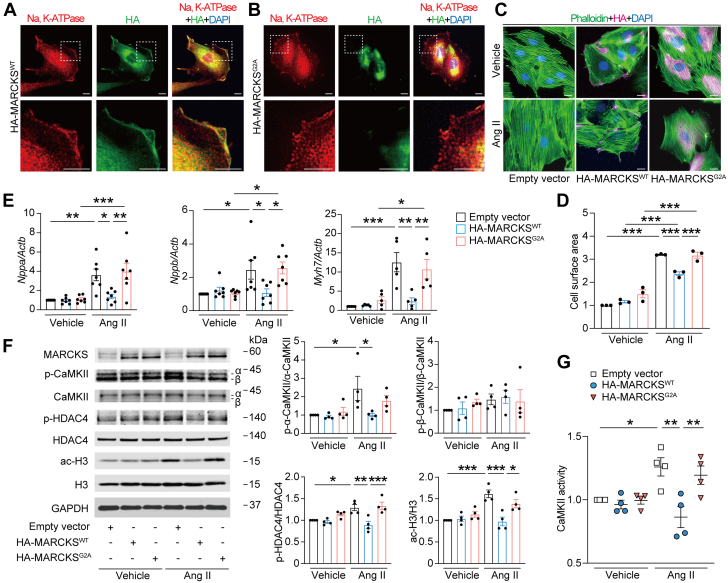
(A and B) Immunofluorescence images for subcellular localization of MARCKS. H9c2 myocytes were transfected with hemagglutinin (HA)-tagged wild-type MARCKS (MARCKSWT) or HA-tagged mutant MARCKS with replacement of N-terminal glycine to alanine (MARCKSG2A) for 48 hours. Cells were stained with anti-Na, potassium–adenosine triphosphatase (K-ATPase) (red), and anti-HA (green) antibodies with DAPI (blue). Images in boxed areas at higher magnification are shown in lower panels. Scale bars = 5 μm. (C) Immunofluorescence images for assessment of myocyte hypertrophy. Transfected H9c2 myocytes with empty vector, MARCKSWT, or MARCKSG2A were stimulated with vehicle or Ang II (1 μmol/L) for 24 hours and stained with phalloidin (green), anti-HA (magenta) antibody, and DAPI (blue). Scale bar = 5 μm. (D) Quantitative analysis of the cell surface area determined by phalloidin staining. Data are expressed as a relative ratio to empty vector with vehicle from 3 independent experiments. (E) Messenger RNA expression levels in Nppa, Nppb, and Myh7. The data were normalized to Actb levels (n = 5-7 in each). (F) Immunoblot analysis for Ca2+/calmodulin-dependent protein kinase II (CaMKII) phosphorylation, histone deacetylase 4 (HDAC4) phosphorylation, and histone H3 acetylation in H9c2 myocytes. The ratios of phosphorylated α-CaMKII (p-α-CaMKII) to total α-CaMKII, phosphorylated β-CaMKII (p-β-CaMKII) to total β-CaMKII, phosphorylated HDAC4 (p-HDAC4) to total HDAC4, and acetylated H3 (ac-H3) to total H3 are quantified and presented in the graphs (n = 4 in each). (G) CaMKII activity. Forty-eight hours after transfection followed by Ang II, cell lysates were collected, and activities of CaMKII were determined and expressed as a relative ratio over the control group of empty vector with vehicle (n = 4 in each). All data are presented as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 by 1-way analysis of variance with Tukey’s post hoc analysis.